Compound and application thereof
A compound and drug technology, applied in the field of drug synthesis, can solve the problems of stimulating leukocyte adhesion, cardiovascular complications, reducing blood flow of gastric mucosa, etc., and achieve the effect of inhibiting inflammatory response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
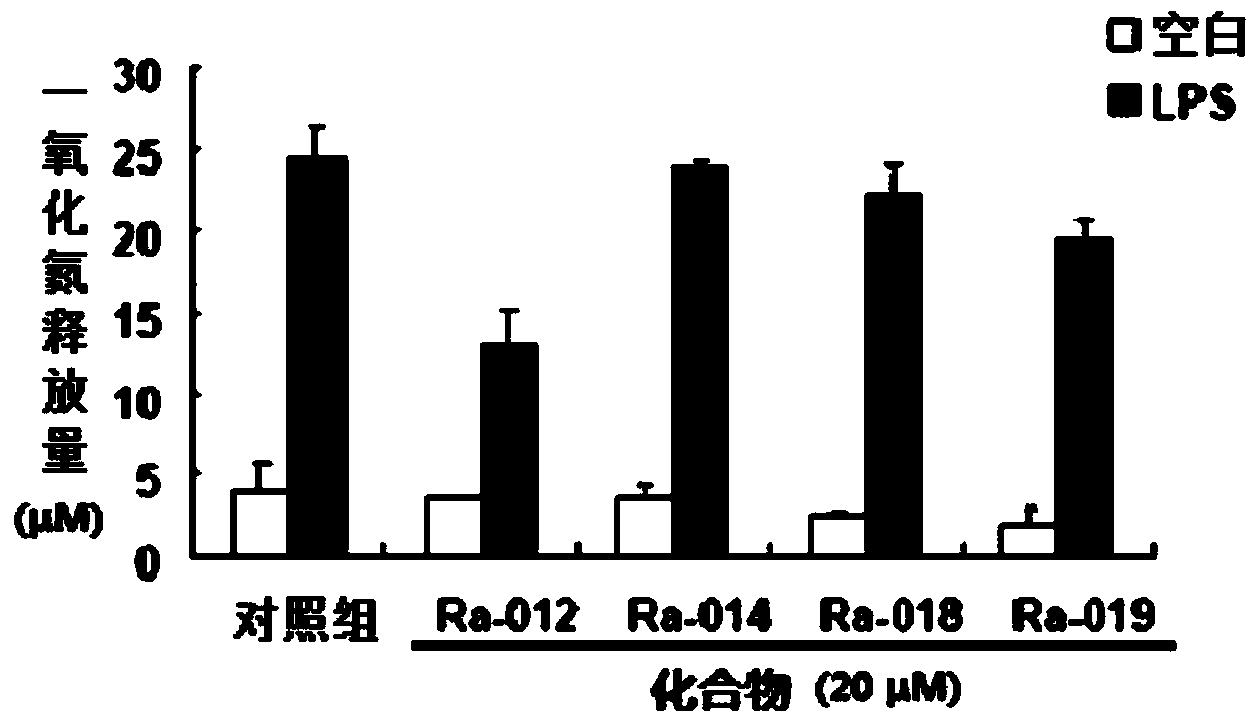
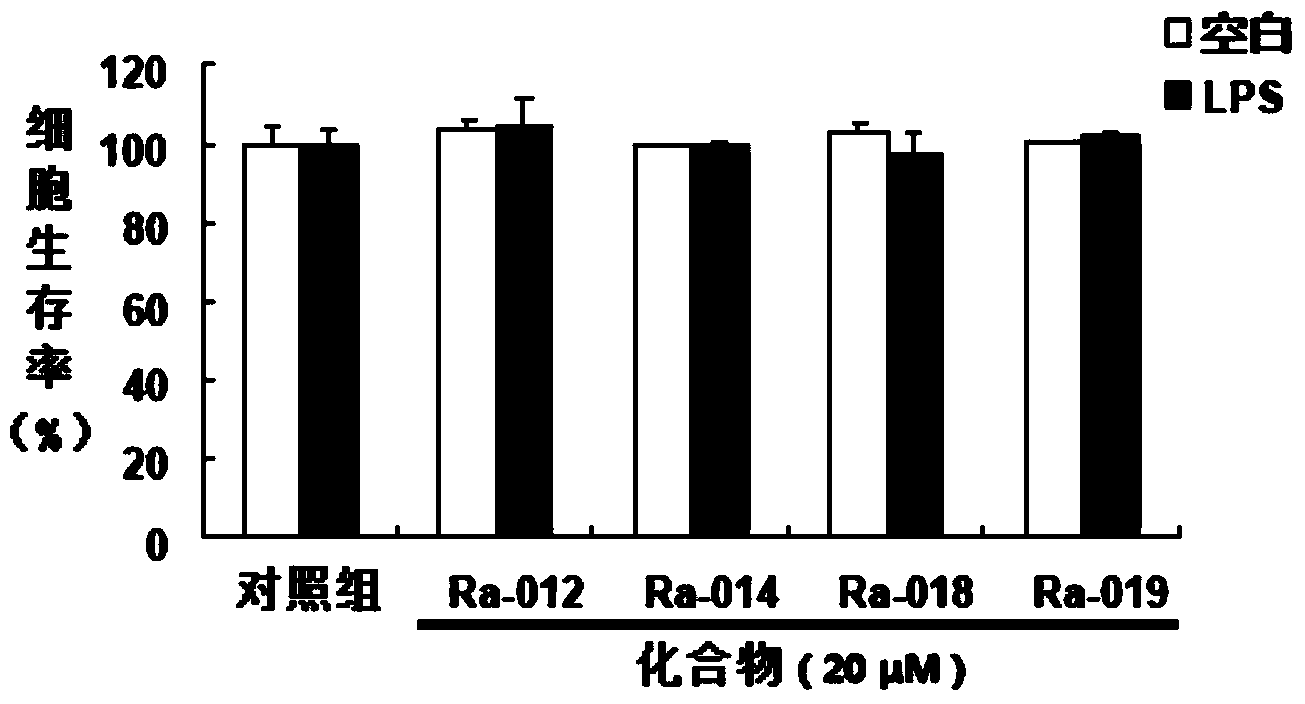
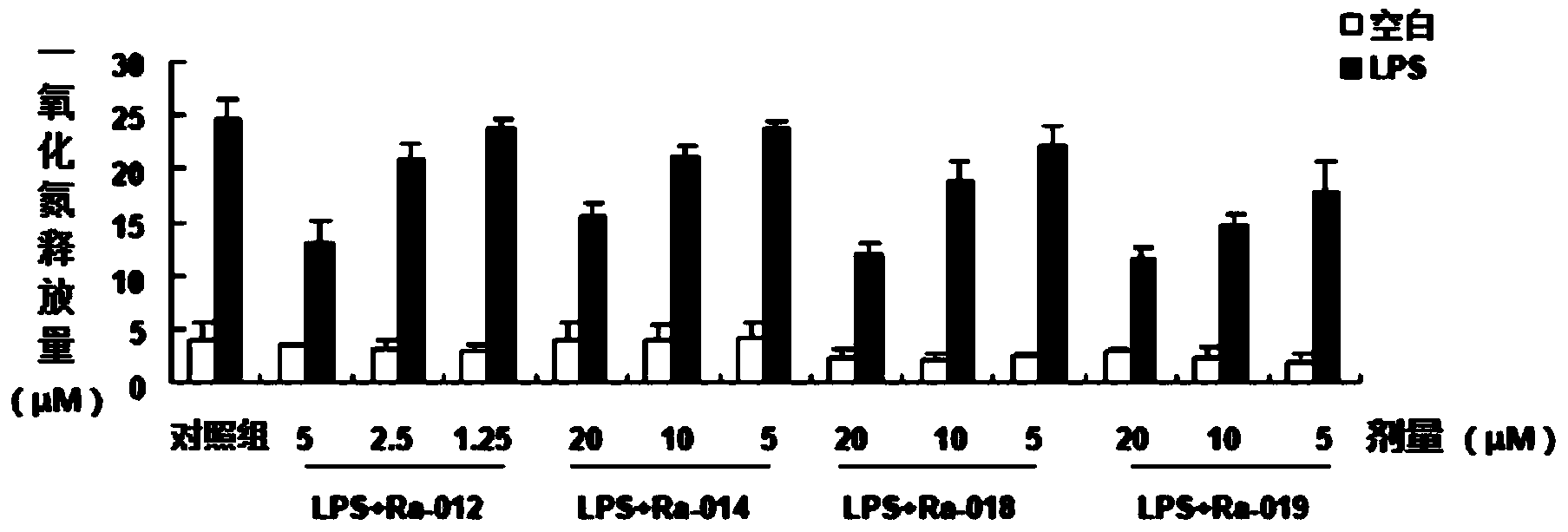
Image
Examples
Embodiment 1
[0047] The present embodiment provides a kind of compound, and the flow process of its synthesis is as follows:
[0048]
[0049] 1) Synthesis of intermediate Ra-012-1
[0050] Cysteine hydrochloride Ra-016 (2.0g, 44mmol) and Boc anhydride (3.5g, 16mmol) were dissolved in dichloromethane (20mL), and triethylamine (2.5mL, 18mmol) was slowly added in an ice-water bath, After the addition, it was raised to room temperature for 16 hours of reaction, washed with 0.5N HCl and saturated sodium chloride successively, and the organic phase was dried and spin-dried to obtain a colorless oil (2.7 g, yield 95%). The colorless oil obtained through NMR analysis is Ra-012-1. The NMR data are as follows: 1 H NMR (400MHz, CDCl 3 )δ4.93(br s,1H),3.32-3.28(m,2H),2.64(t,J=6.8Hz,2H),6.43(s,1H),6.25(d,J=8.0Hz,1H) ,1.44(s,9H).
[0051] 2) Synthesis of intermediate Ra-012-2
[0052] Under an ice-water bath, diclofenac Ra-009 (100 mg, 0.34 mmol) and Ra-012-1 were dissolved in dichloromethan...
Embodiment 2
[0056] The present embodiment provides a kind of compound, and the flow process of its synthesis is as follows:
[0057]
[0058] Synthesis of product Ra-014:
[0059] Dissolve Ra-009 (50mg, 1.7mmol) in 10mL of dichloromethane and 10mL of dioxane, add Ra-015 (770mg, 3.4mmol) and dicyclohexylcarbodiimide (420mg, 3.4mmol) under ice-cooling and 4-dimethylaminopyridine (700mg, 3.4mmol). Stir the reaction at room temperature for 24 hours, filter with diatomaceous earth, and concentrate under reduced pressure to obtain a white solid (260 mg, yield 29%) by silica gel column chromatography. The white solid was determined by NMR. After analysis, the white solid was Ra-014 . The NMR data are as follows: 1 H NMR(400MHz,DMSO-d6)δ9.79(s,1H),9.67(s,1H),7.54(d,J=8.0Hz,2H),7.40(d,J=8.4Hz,2H),7.31 (d,J=6.8Hz,1H),7.22(t,J=8.0Hz,1H),7.13-7.06(m,2H),7.03(d,J=16.0Hz,1H),6.93-6.86(m, 2H), 6.82-6.74(m, 4H), 6.43(s, 1H), 6.25(d, J=8.0Hz, 1H), 4.07(s, 2H).
Embodiment 3
[0061] The present embodiment provides a kind of compound, and the flow process of its synthesis is as follows:
[0062]
[0063] 1) Synthesis of intermediate Ra-018-1
[0064] Dissolve Ra-009 (1.0g, 3.3mmol) in 20mL of dichloromethane and 20mL of water, add sodium bicarbonate (1.05mg, 1.25mmol) and tetrabutylammonium bisulfate (110mg, 0.33mmol), and stir for 5min, Chloromethyl chlorosulfonate (600 mg, 3.63 mmol) was dissolved in 10 mL of dichloromethane and added dropwise to the above solution, stirred at room temperature for 1 hour, extracted with dichloromethane (30 mL) and water (20 mL) for 3 Once, combine the organic phases, dry over anhydrous sodium sulfate, concentrate under reduced pressure, add 20 mL of acetone to dissolve, add sodium iodide (2.0 g, 13.2 mmol), stir at room temperature for 24 hours, spin off the acetone, add 20 mL of dichloromethane, Celite was filtered, and the organic layer was concentrated under reduced pressure to obtain a yellow solid (1.3 g,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com