Polypeptide for enrichment, separation and detection of circulating tumor cells and application thereof
A tumor cell and polypeptide solution technology, which is used in the enrichment, separation and detection of circulating tumor cell polypeptides and its application field, can solve the problem of high cost of detecting CTCs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1. Verification of polypeptide-specific recognition of EpCAM
[0109] 1. The sequence of the peptide used, reagents and verification cells
[0110] Peptide fragments (synthesized by Shanghai Keyept Biotechnology Co., Ltd., with a purity of 98 mass%) are shown in Table 1 below. Magnetic nanoparticles: streptavidin-magnetic nanoparticles (solulink, ST022912, USA).
[0111] Table 1
[0112]
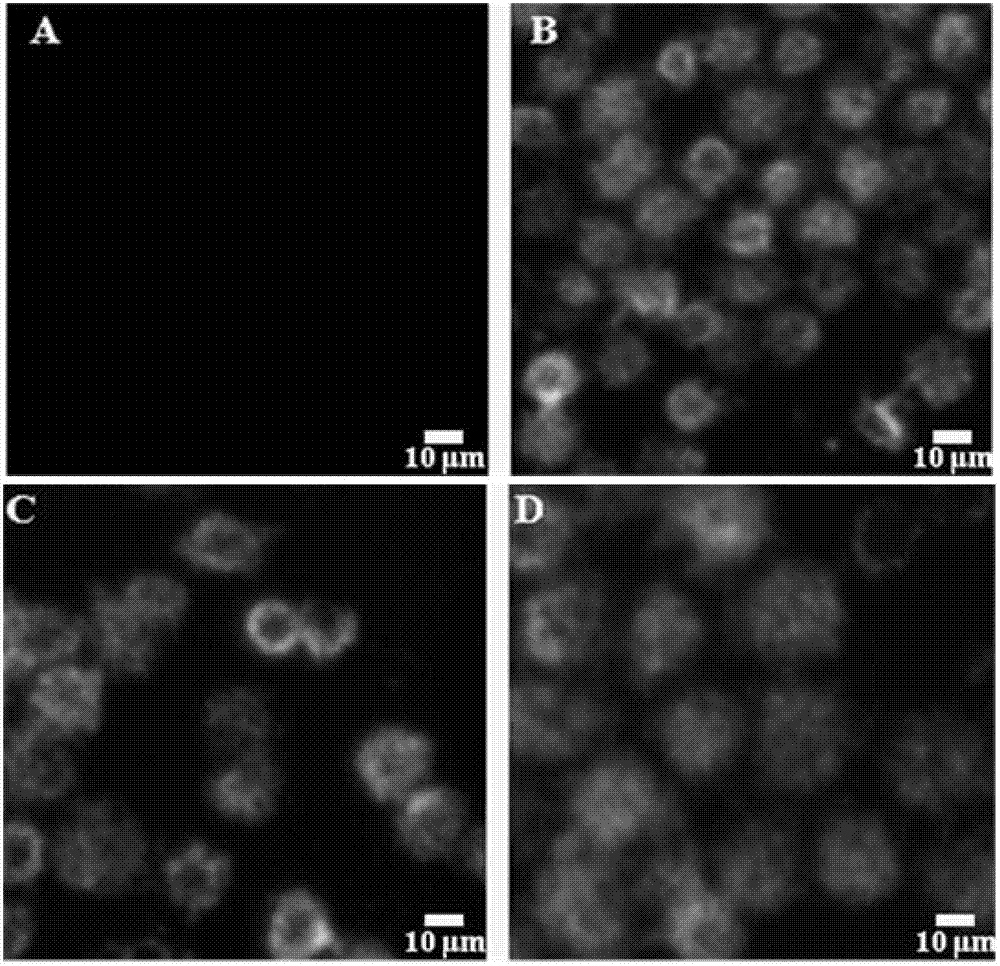
[0113] MCF-7 cells, which are known to be consistent with reports, were regarded as EpCAM-positive cells, and HL-60 cells were regarded as EpCAM-negative cells to verify the specific recognition of the polypeptide fragment of EpCAM.
[0114] 2. Specific method
[0115] 1) Verification of EpCAM-positive specific peptides.
[0116] Firstly, a series of FITC-peptides designed and synthesized were made into 1 mg / ml mother solution with PBS with pH=7.2; a certain amount (≥10 6 1) MCF-7 and HL-60 cells in a 1.5 μl centrifuge tube, add 7 μl of each polypeptide mother soluti...
Embodiment 2
[0127] Example 2. Specific recognition of polypeptides Pep7-2 and Pep7-3 by biotin-streptavidin Interaction and magnetic nanoparticle coupling
[0128] 1. Take 250 μl of 5 mg / ml magnetic nanoparticles, mix them on a vortex mixer (WH-866, Taicang Hualida Experimental Equipment Co., Ltd.) for 3~5 minutes, collect them with a strong magnetic magnet, and remove the solvent. Then take 500 μl of 1 mg / ml FITC-Pep7-2-biotin (Biotin) and 1 mg / ml FITC-Pep7-3-biotin (Biotin) in PBS and mix them with magnetic nanoparticles (polypeptides and magnetic nanoparticles The mass ratio of the particles is 2:5), and then placed on a shaker (THZ-D, Taizhou Wanda Gas Spring Co., Ltd.) and mixed for 40min at 25°C and 150rpm. Then use a strong magnetic magnet to enrich the magnetic nanoparticles on one side of the centrifuge tube, absorb the liquid from the other side of the centrifuge tube after about 10 minutes, then add a certain amount of PBS buffer, resuspend the magnetic nanoparticles, enric...
Embodiment 3
[0130] Example 3. Characterization of polypeptide-magnetic nanoparticles
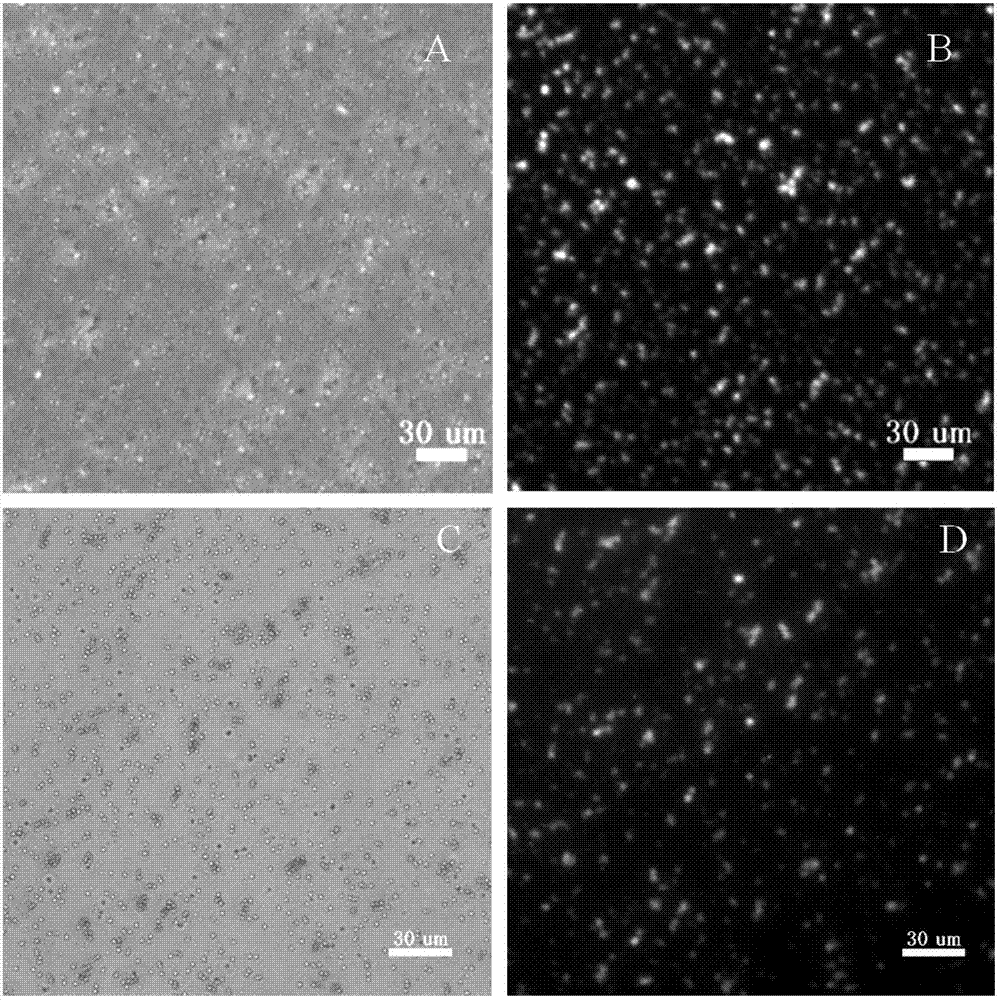
[0131] Take the FITC-Pep7-2-biotin (Biotin)-magnetic nanoparticle and FITC-Pep7-3-biotin (Biotin)-magnetic nanoparticle solution prepared in 1 in Example 2 (the mass of the polypeptide and the magnetic nanoparticle The ratio is 2:5) a little, take pictures under the microscope, the result is as follows figure 2 As shown, A and B are photos of Pep7-2-magnetic nanoparticles under bright field and blue light excitation respectively, and the magnetic nanoparticles modified with Pep7-2 emit green light under blue light (the absorption and emission wavelengths of FITC are 495nm and 535nm); C and D are the photos of Pep7-3-magnetic nanoparticles excited by bright field and blue light, respectively, and the magnetic nanoparticles modified with pep7-3 emit green light under blue light; indicating that the peptide and magnetic nanoparticles are successfully coupled .
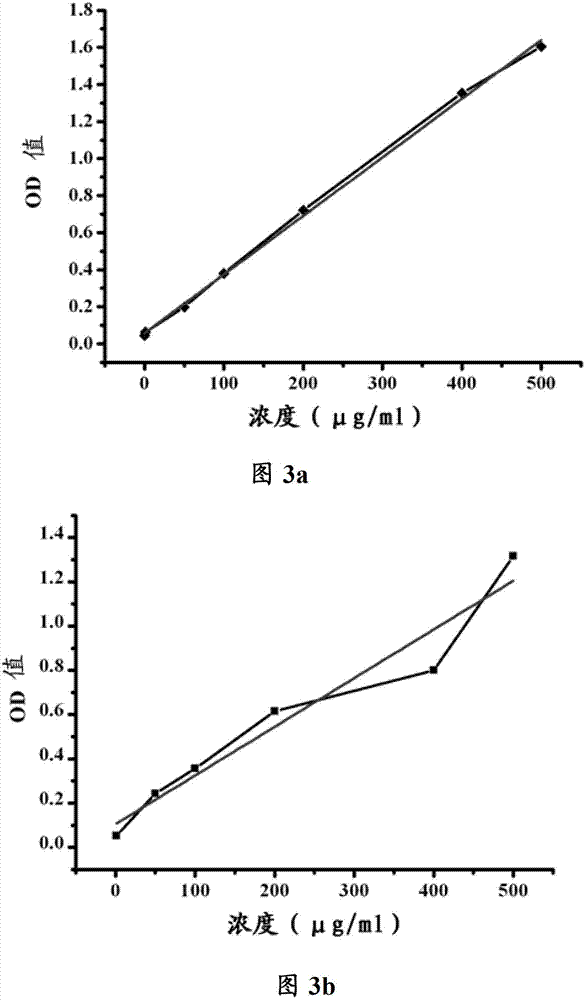
[0132] Use FITC-Pep7-2-biotin (Biotin) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com