Cationic liposome, and preparation method and application thereof
A cationic liposome and carrier technology, which is applied to other methods of inserting foreign genetic materials, pharmaceutical formulas, genetic material components, etc., can solve the problems of low transfection efficiency, achieve optimized preparation parameters, improve transfection efficiency, and use Dose Reduction Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The present invention also relates to a preparation method of cationic liposome, comprising the following steps:
[0050] Step 10, take 1 weight part of DOPE, 1 weight part of DC-Chol, and 2.5 weight parts of OQCMC according to the proportion and dissolve them in the chloroform solvent;
[0051] Step 11, mix the solution obtained in step 10, add ultrapure water for blending, and sonicate the probe for 10 minutes;
[0052] Step 12, remove the residual solvent of the product obtained in step 11 in a rotary evaporator, and obtain it in a vacuum state; the evaporation temperature of the rotary evaporator is 35°C.
[0053] The present invention also relates to a cationic liposome complex. The cationic liposome complex uses cationic liposome as an encapsulation layer, and a drug carrier, a gene carrier, or a gene and a drug are co-loaded in the encapsulation layer. At least one of the carriers; the cationic liposome is prepared from the following components in parts by weigh...
Embodiment 1
[0061] Embodiment one cationic liposome formula of the present invention and preparation technology are preferred
[0062] Cationic liposomes were selected as the target liposome type, and after preliminary screening tests, octadecyl quaternary ammonium carboxymethyl chitosan (OQCMC), dioleoylphosphatidylethanolamine (DOPE) and 3-β-N-N '-N'-Dimethylaminoethyl-carbamoylcholesterol (DC-Chol), as the base ingredient, was then optimized for formulation.
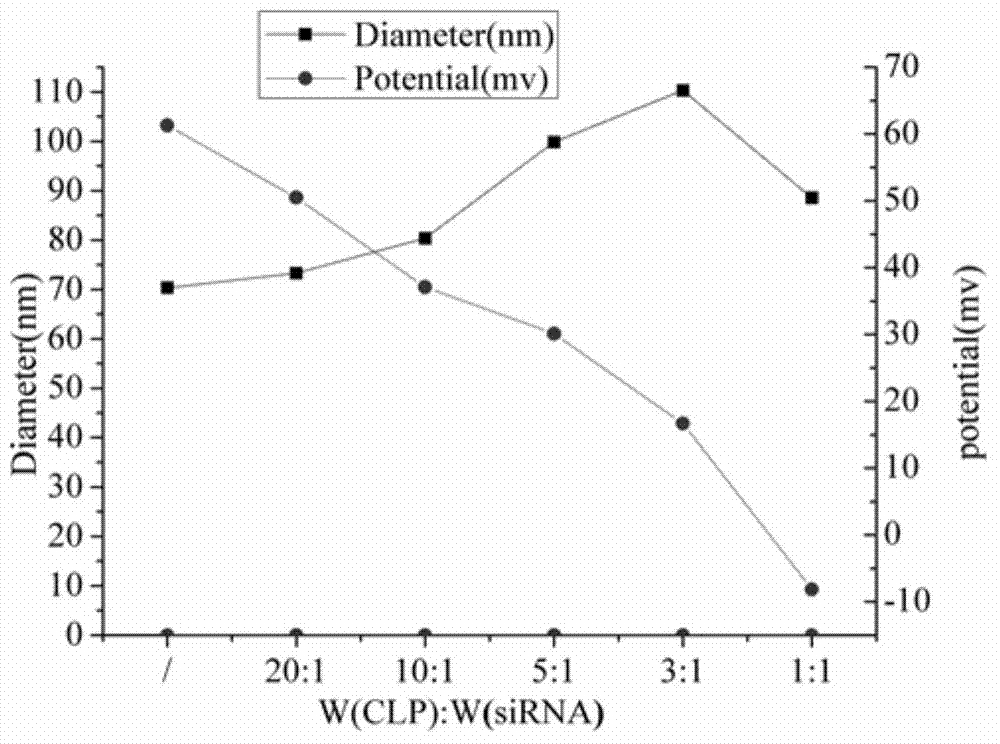
[0063] The cationic liposome preparation method of the present invention and the amount of CLP and siRNA are optimized according to a certain ratio, as shown in Table 1 (the particle size and potential change of the complex caused by the mass ratio of the two): CLP-siRNA increases in size with the increase of siRNA mass. The size and charging conditions change, showing that the particle size increases continuously, while the potential decreases continuously. This indirectly shows that siRNA and CLP have an electrostatic binding ...
Embodiment 2
[0066] The preparation of embodiment two cationic liposome CLP of the present invention
[0067] Weigh 2.5 parts by weight of OQCMC, 1 part by weight of DOPE, and 1 part by weight of DC-Chol, and dissolve them in chloroform; The residual chloroform was removed by an instrument to obtain OQCMC-CLP (abbreviated as CLP) and CLP-PTX with a concentration of 2.67 mg / mL (V / W). The samples were sealed, protected from light, and stored at a low temperature of 4°C for later use.
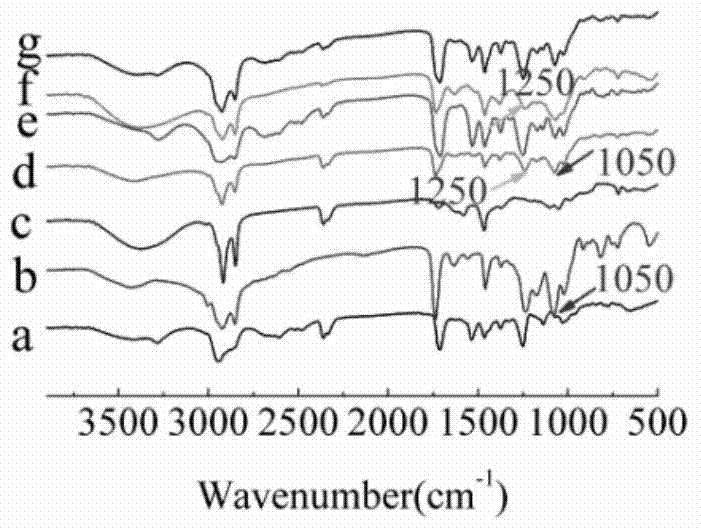
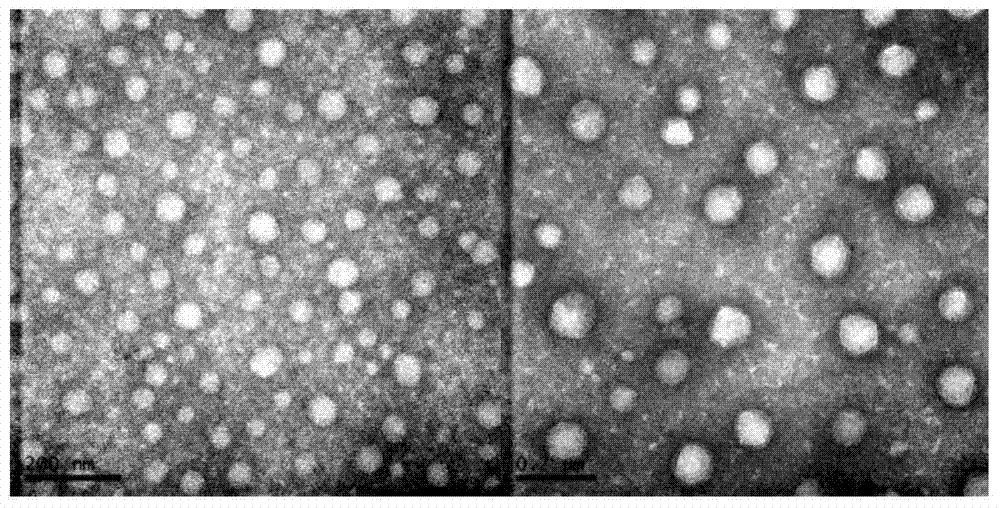
[0068] The physical and chemical properties of the prepared cationic liposomes were characterized by infrared spectroscopy (FTIR), Malvern particle size analyzer, TEM, and AFM for blank CLP and CLP complexes. Its infrared spectrum is as figure 1 As shown, CLP was successfully prepared from DC-Chol, DOPE and OQCMC. In the DC-Chol-DOP spectrum, at 1050cm -1 A new absorption peak (-P=O-group) appeared at , which corresponds to the carboxyl group of DOPE, proving that DOPE was attached to DC-Chol. At the same...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com