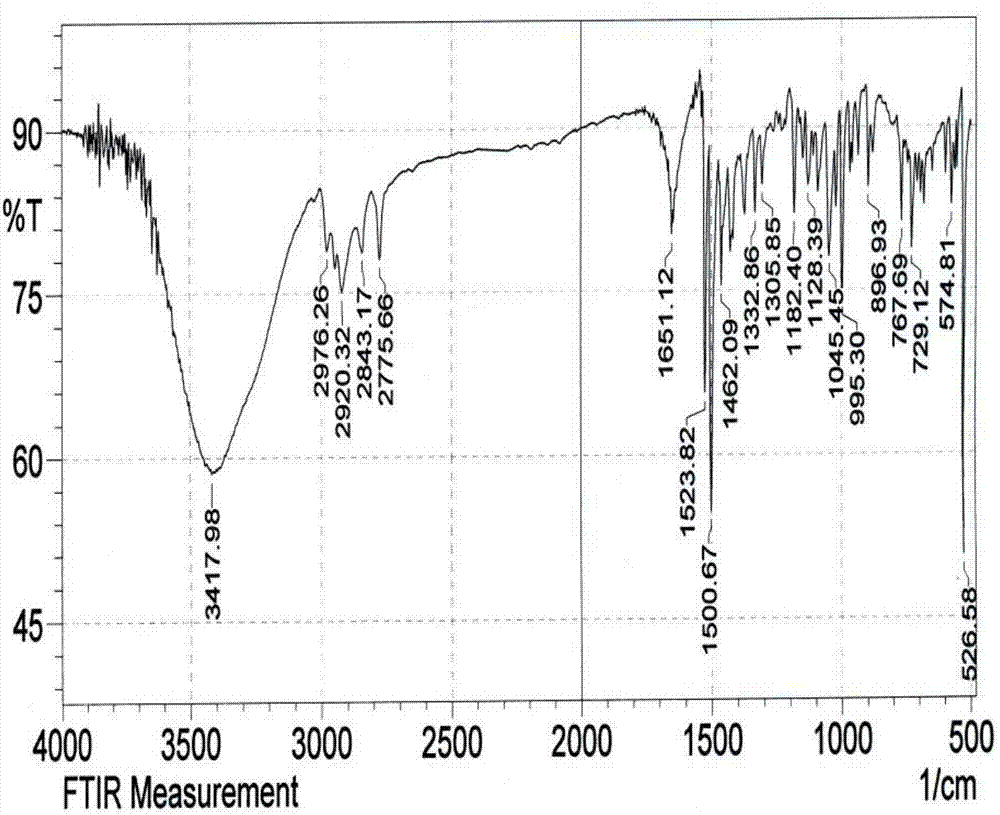
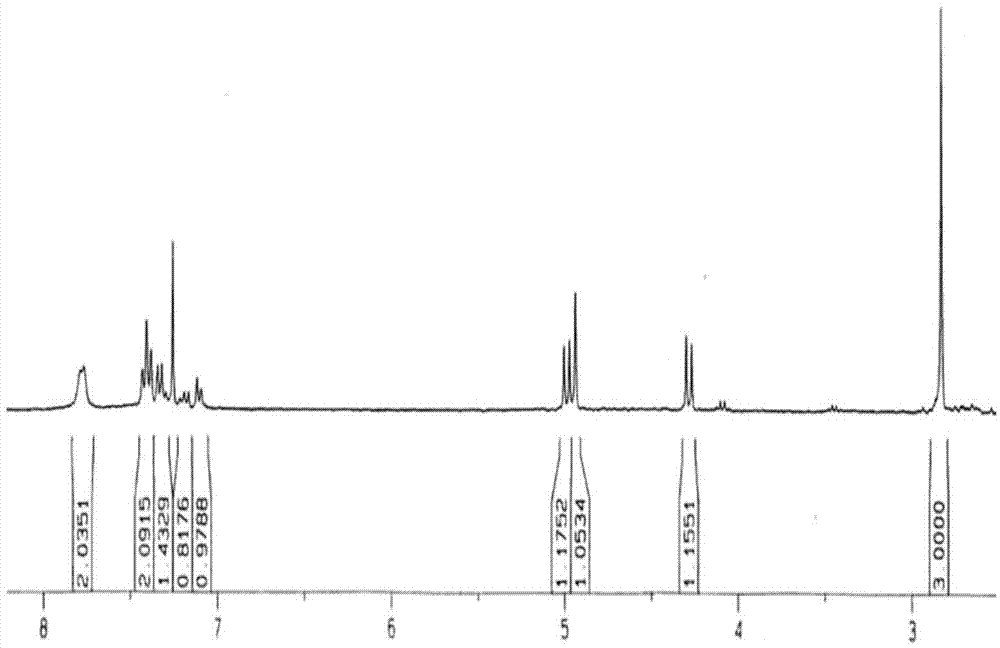
Preparation method and application of micron acicular-structure N-methyl-2-phenyl-3,4-fullerenylpyrrolidine
A technology of fullerenyl pyrrolidine and micron needles, which is applied in the field of preparation of fullerene derivatives, can solve the problems of low product yield, low catalytic conversion rate, and low purity, and achieve high activity and long life. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 (S1)
[0031] The preparation method of N-methyl-2-phenyl-3,4-fullerenylpyrrolidine, its step comprises:
[0032] (1) Under the protection of argon, the fullerene C 60 Dissolve in freshly distilled toluene and stir for 1 h to give fullerene C 60 Toluene solution;
[0033] (2) to the fullerene C 60 Add freshly distilled benzaldehyde and sarcosine to the toluene solution, and place it under reflux at 120°C for 2 hours to obtain a reaction solution, wherein, during the reaction, the three raw materials fullerene C 60 , The molar ratio of benzaldehyde and sarcosine is 1:6:4;
[0034] (3) Pass argon gas into the reaction solution, after it is cooled to room temperature, it is filtered, concentrated and separated by column chromatography to obtain a reaction concentrate;
[0035] (4) Use cyclohexane to wash out the fullerene C that has not participated in the reaction in turn for the reaction concentrate 60 , and then rinse the residue with an eluent that is a...
Embodiment 2
[0036] Example 2 (S2)
[0037] The preparation method of N-methyl-2-phenyl-3,4-fullerenylpyrrolidine, its step comprises:
[0038] (1) Under the protection of argon, the fullerene C 60 Dissolved in freshly distilled toluene and stirred for 2 h to give fullerene C 60 Toluene solution;
[0039] (2) to the fullerene C 60 Add freshly distilled benzaldehyde and sarcosine to the toluene solution, and place it under reflux at 120°C for 3 hours to obtain a reaction solution, wherein, during the reaction, the three raw materials fullerene C 60 , The molar ratio of benzaldehyde and sarcosine is 1:6:4;
[0040] (3) Pass argon gas into the reaction solution, after it is cooled to room temperature, it is filtered, concentrated and separated by column chromatography to obtain a reaction concentrate;
[0041] (4) Use cyclohexane to wash out the fullerene C that has not participated in the reaction in turn for the reaction concentrate 60 , and then rinse the residue with an eluent that ...
Embodiment 3
[0063] Example 3 (M3)
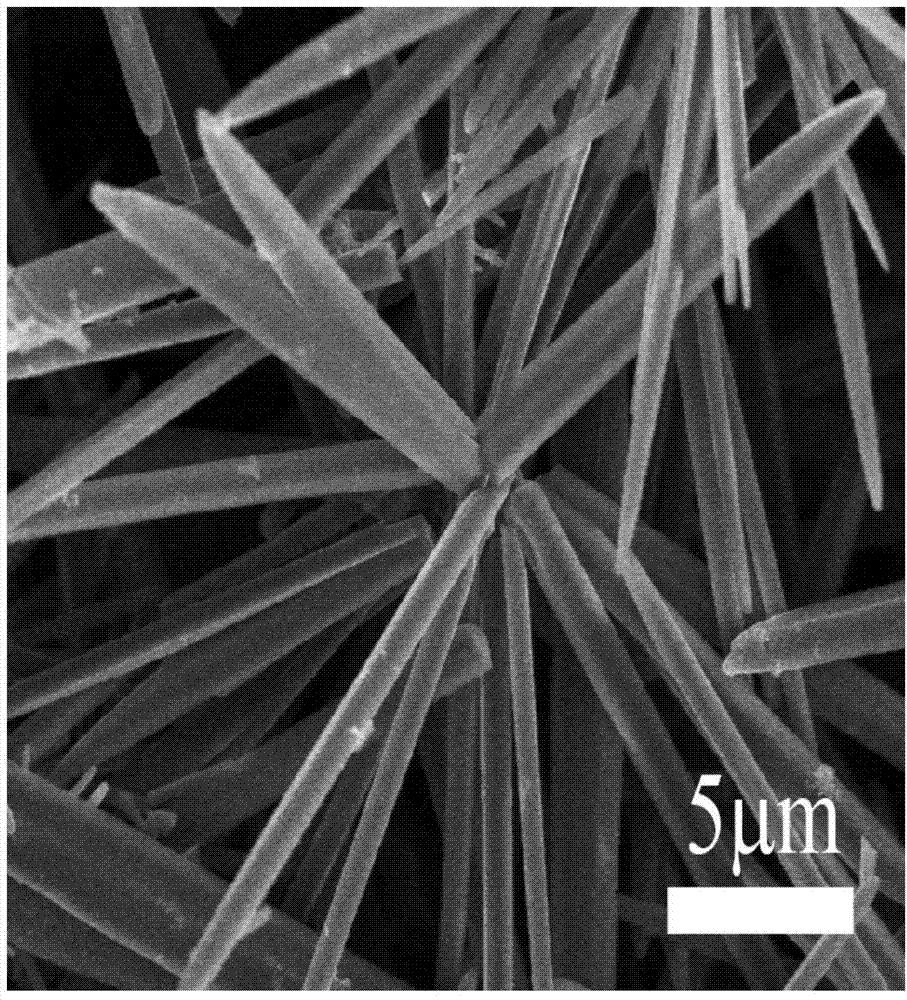
[0064] The N-methyl-2-phenyl-3,4-fullerenylpyrrolidine obtained in Example 1 was treated with toluene as a solvent, and the N-methyl-2-phenyl-3, 4-Fullerenylpyrrolidine is dissolved in toluene, and its concentration in toluene is 0.75mgmg / mL, and then a surfactant is added, wherein the surfactant is prepared by dissolving sodium dodecylsulfonate in methanol , the substance concentration of the surfactant is 3mmol / L; the volume ratio of methanol to toluene is 5:1, the temperature is 15°C, and the magnetic stirring time is 20min. After centrifugation, the speed of the centrifuge is 5000r / min, the centrifugation time is 10min, remove the upper layer solution, wash 3 times with absolute ethanol, after centrifugation, place it at room temperature and dry for 10min, you can get micron N-methyl-2-phenyl-3,4-fullerenylpyrrolidine with needle-like structure.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com