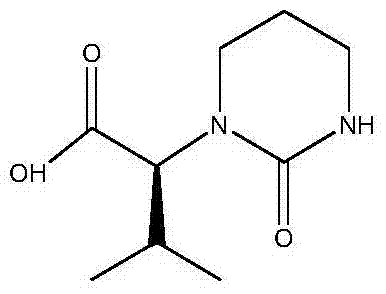
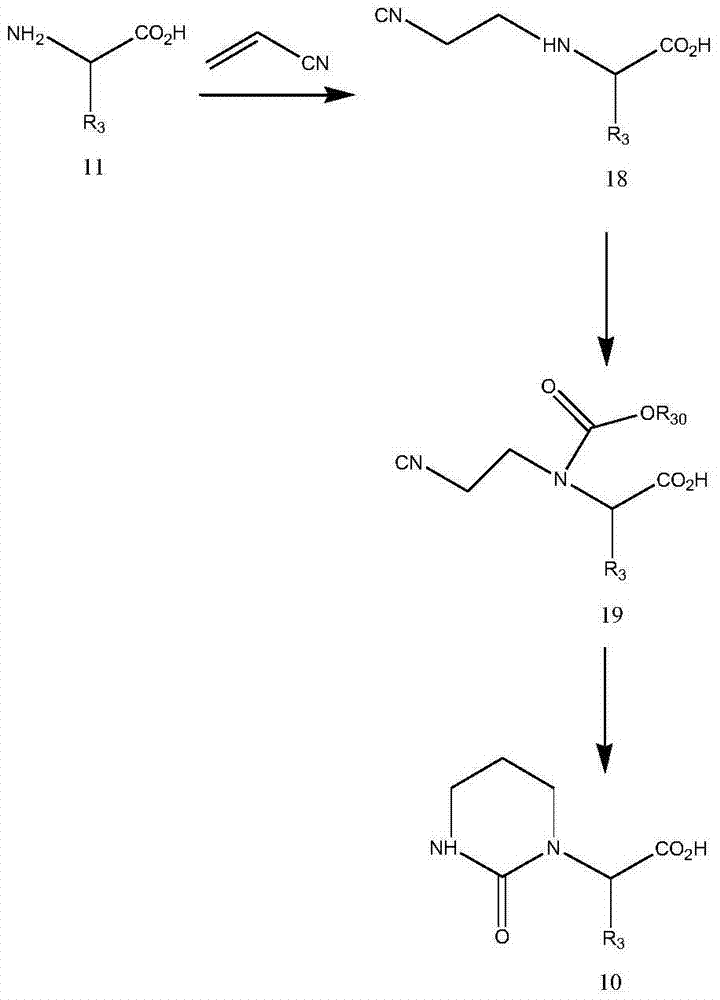
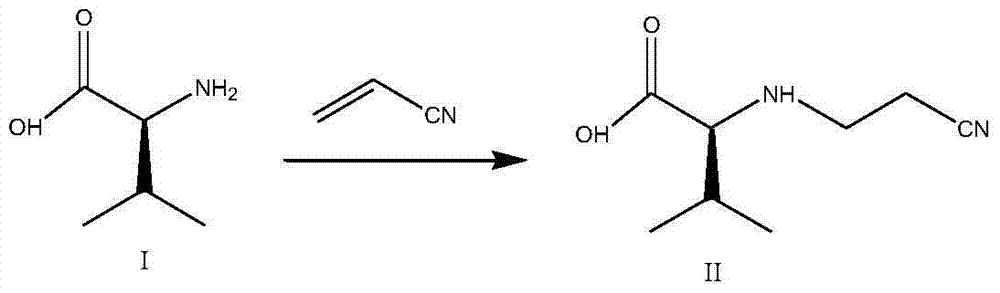
Preparation method of 2S-(1-tetrahydropyramid-2-one)-3-methylbutanoic acid
A technology of ectoine and methylbutyric acid, applied in the direction of organic chemistry, can solve problems affecting the health of production personnel, polluting the environment, complicated procedures, etc., to save complicated procedures, maintain the production environment, and save operating time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042]1) Pour purified water (200kg) into the reaction tank, add L-valine (100kg) while stirring, cool down to 20-25°C after the addition is complete, and continue stirring for 15 minutes. 2) Add potassium hydroxide aqueous solution (57kgKOH mixed with 57kg water) dropwise, cool down to 20-25°C after the addition is complete, and continue stirring for 30 minutes until clarification. 3) Cool the solution to 0-5°C and add acrylonitrile (50kg) dropwise. After the addition is complete, keep the temperature at 0-5°C and react for 5 hours. The concentration of L-valine detected by TLC was 2.0%. 4) Add purified water (250kg). After the addition is complete, heat up to 10-15°C, and adjust the pH value to 10.0 with concentrated hydrochloric acid. 5) Slowly add methyl chloroformate (161kg) dropwise, and at the same time add 30% sodium hydroxide The pH value of the solution was maintained at 10.0. After the feeding was completed, the temperature was maintained at 10-15° C. and the pH va...
Embodiment 2
[0044] 1) Pour purified water (200kg) into the reaction tank, add L-valine (100kg) while stirring, cool down to 20-25°C after the addition is complete, and continue stirring for 15 minutes. 2) Add potassium hydroxide aqueous solution (57kgKOH mixed with 57kg water) dropwise, cool down to 20-25°C after the addition is complete, and continue stirring for 30 minutes until clarification. 3) Cool the solution to 0-5°C, add acrylonitrile (50kg) dropwise, and keep the temperature at 0-5°C for 7 hours after the addition is complete. The concentration of L-valine detected by TLC was 1.0%. 4) Add purified water (250kg). After the addition is complete, heat up to 10-15°C, and adjust the pH value to 10.0 with concentrated hydrochloric acid. 5) Slowly add methyl chloroformate (161kg) dropwise, and at the same time add 30% sodium hydroxide The pH value of the solution was maintained at 10.0. After the feeding was completed, the temperature was maintained at 10-15° C. and the pH value was m...
Embodiment 3
[0050] 1) Pour purified water (200kg) into the reaction tank, add L-valine (100kg) while stirring, cool down to 20-25°C after the addition is complete, and continue stirring for 15 minutes. 2) Add potassium hydroxide aqueous solution (57kgKOH mixed with 57kg water) dropwise, cool down to 20-25°C after the addition is complete, and continue stirring for 30 minutes until clarification. 3) Cool the solution to 0-5°C and add acrylonitrile (50kg) dropwise. After the addition is complete, keep the temperature at 0-5°C and react for 6 hours. The concentration of L-valine detected by TLC was 1.6%. 4) Add purified water (250kg). After the addition is complete, heat up to 10-15°C, and adjust the pH value to 10.0 with concentrated hydrochloric acid. 5) Slowly add methyl chloroformate (161kg) dropwise, and at the same time add 30% sodium hydroxide The pH value of the solution was maintained at 10.0. After the feeding was completed, the temperature was maintained at 10-15° C. and the pH v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com