Biotin labeled ligustrazine and preparation method thereof
A biotin-labeled, ligustrazine technology, applied in the biological field, achieves the effects of protecting and stabilizing, promoting recovery and reducing the accumulation of free radicals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
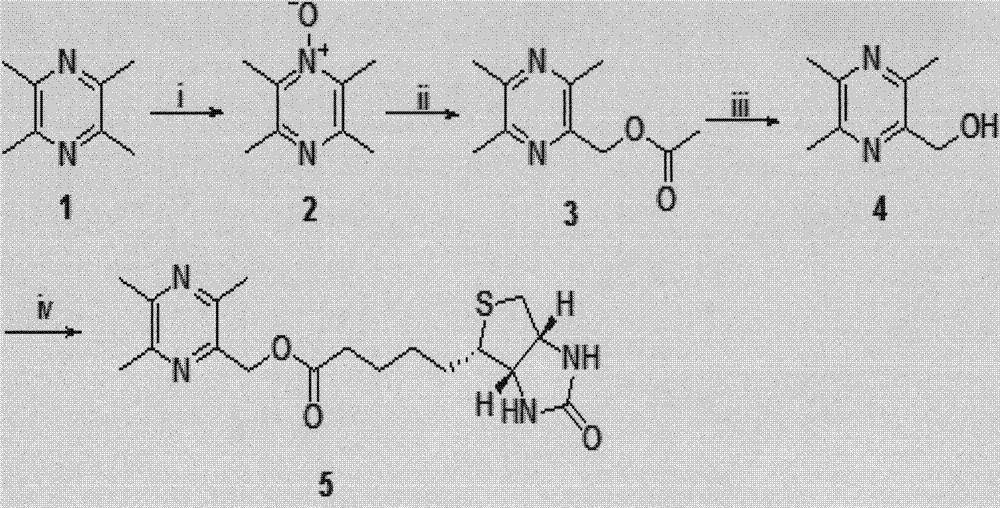
[0043] Example 1 Preparation of biotin-labeled Ligustrazine: first Ligustrazine is oxidized to 2-hydroxyl-3,5,6-trimethylpyrazine, and D-biotin, EDCI, and DMAP according to 12:29:30 Dissolve in anhydrous DMF at a mass ratio of : 1, stir and react at 60°C for 72h, monitor the completion of the reaction by TLC, pour the reaction solution into 30mL of ether, stir at 4°C for 4h, a white precipitate precipitates, filters, washes with ether, The crude product was purified by column chromatography to obtain biotin-labeled ligustrazine with a yield of about 78%.
[0044] (1) Synthesis of 2-hydroxy-3,5,6-trimethylpyrazine, namely intermediate
[0045] With 6.8g, the ligustrazine that molar concentration is 50mmoL, i.e. substance 1, 12mL glacial acetic acid and 6mL mass concentration are 30% H 2 o 2 Add it into a three-necked flask, heat and react at 70°C for 4 hours, then add 6 mL of 30% H 2 o 2 , Continue to reflux for 4h. TLC monitors that the reaction is complete, cool to room ...
Embodiment 2
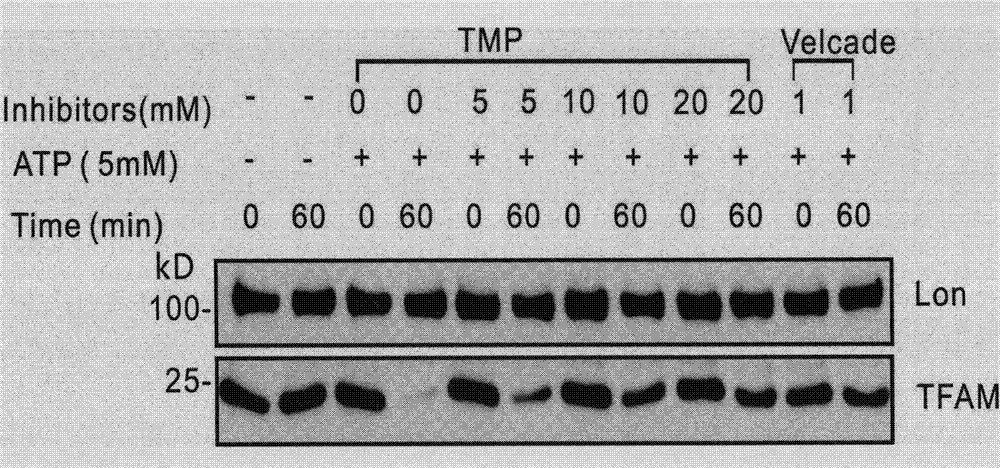
[0051] Example 2 Ligustrazine inhibits the degradation of TFAM in vitro
[0052] Use 20 μM bortezomib (Velcade) as a positive control, DMSO as a negative control, and 20 μL of 0.4 mM ligustrazine for doubling dilution; take the centrifuge tube for labeling, add reaction buffer, Lon, BSA, TMP (DMSO or Velcade), mix thoroughly; incubate at 37°C for 1 hour; add TFAM and ATP in turn to make the final concentration of Lon, TFAM, and BSA 300nM, 150nM, 0.1μg / μL, mix well, and incubate at 37°C for 1h; At the time point of 0 and 60 minutes, draw 20 μL samples from each tube, add 5 μL 5X sample buffer; heat at 95°C for 5 minutes to fully denature the protein; load 8 μl standard protein Maker and 20 μL denatured protein samples to 12% SDS-PAGE, and Western blot analysis.
[0053] The results showed that TFAM remained stable after 1 hour of reaction without adding ATP and was not degraded by Lon; in the positive control group, Velcade inhibited the activity of Lon, and TFAM remained stab...
Embodiment 3
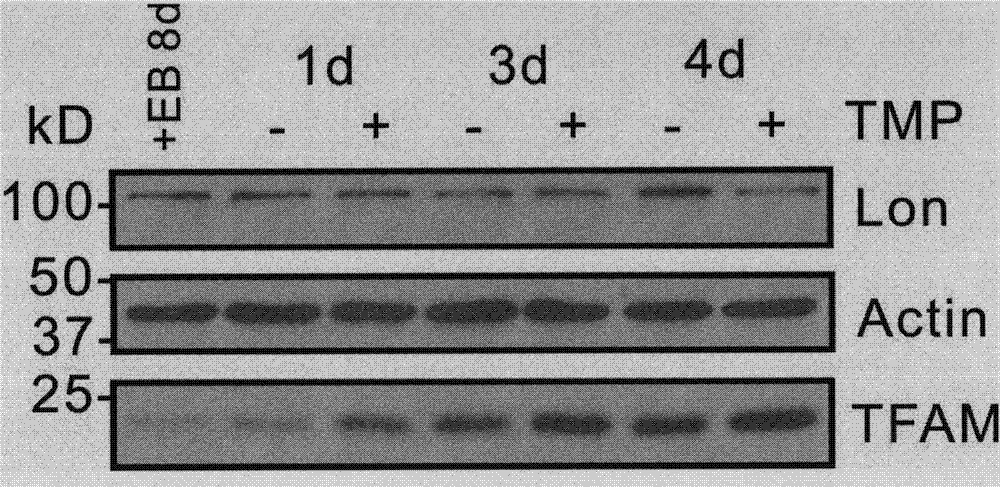
[0054] Ligustrazine in embodiment 3 promotes Hela low Recovery of TFAM in cells
[0055] 1. Cultivate HeLaρ with EB complete medium containing 50 ng / mL + Eight days after the cells, EB was removed, and the cells were divided into two groups. One group was treated with 10M ligustrazine, and the other group was treated with DMSO as a negative control. The cells were harvested at 1, 3, and 4 days, and the protein was extracted and determined by BCA method. After the protein concentration, the protein concentration was adjusted to 1g / μL, heated at 95°C for 5min, and the sample was loaded on 12% SDS-PAGE for western blot analysis ( image 3 , Figure 4 ).
[0056] 2. Treatment of HeLaρ with different concentrations (2.5, 5, 10 μM) of ligustrazine low After 24 hours of cells, no ligustrazine or DMSO was added as a negative control, the protein was extracted, the protein concentration was determined by the BCA method, and the protein concentration was adjusted to 1 μg / μL, heated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com