Human cytochrome P450 3A5 enzyme and NADPH-cytochrome P450 oxidoreductase co-expression system
A cytochrome and reductase technology, applied in the field of genetic engineering, can solve the problems of complex reaction conditions and difficult separation of intermediate products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Construction of human cytochrome P450 3A5 enzyme (CYP3A5) and NADPH-cytochrome P450 oxidoreductase (CYPOR) co-expression system
[0037] The specific steps of construction are as follows:
[0038] Digest the plasmid pBS-2A (containing the 2A peptide gene) with XbaI, recover the 3.0kb backbone fragment and the 130bp 2A connecting peptide respectively, dephosphorylate the 3.0kb backbone fragment, and reconnect to select the reverse clone, namely pBS-2A(- );
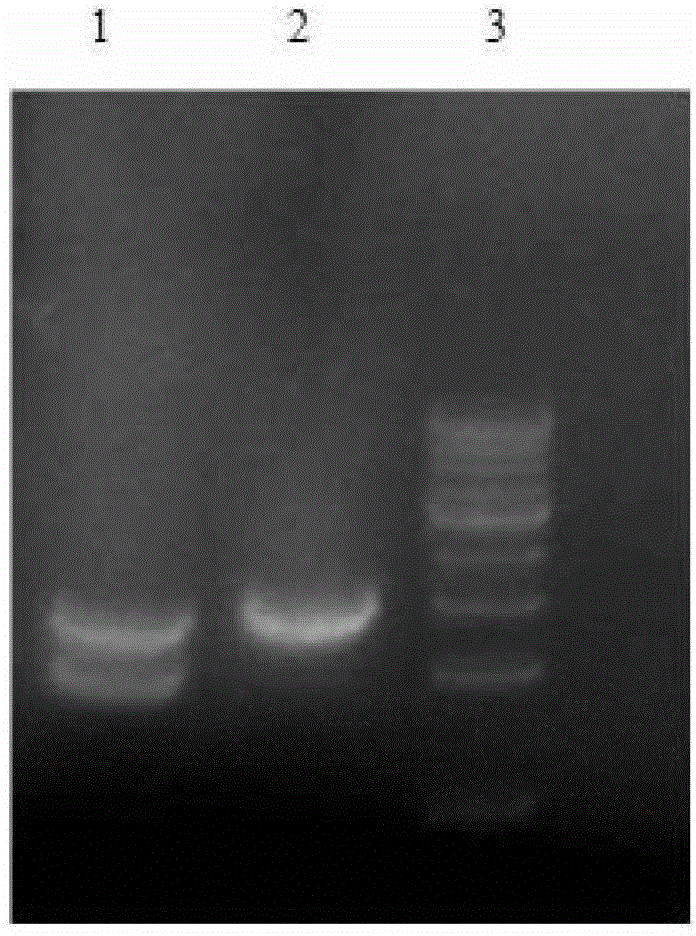
[0039] Insert the NADPH-cytochrome P450 oxidoreductase gene (CYPOR) between the BglII and Not I restriction sites of the vector pBS-2A(-) to obtain the vector pBS-2A-POR (see Figure 14 );
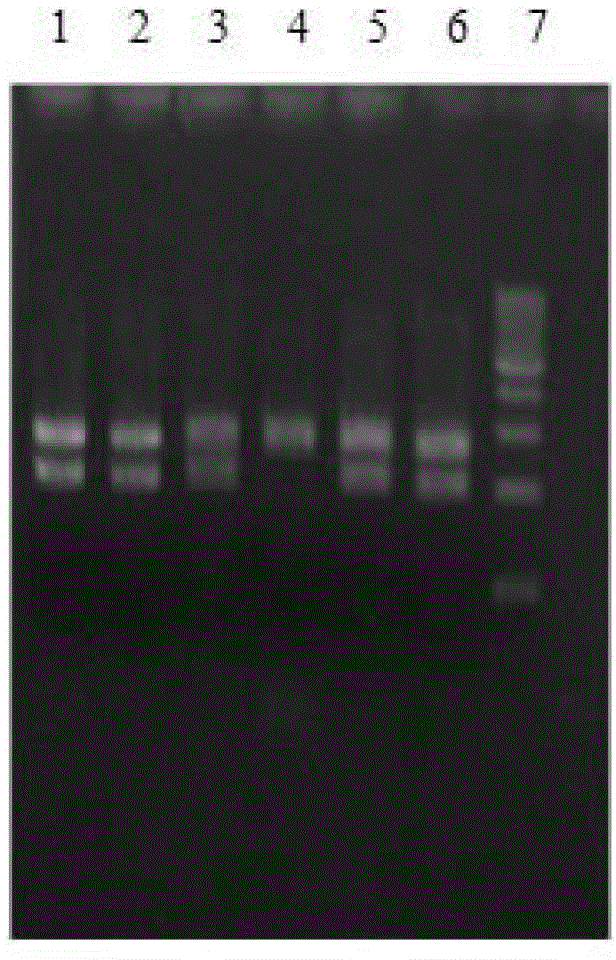
[0040] The human cytochrome P4503A5 enzyme gene was directional inserted between the BamH I and Xba I restriction sites of the vector pBS-2APOR to obtain the vector pBS-CYP3A5-2A-POR (see Figure 5-8 );
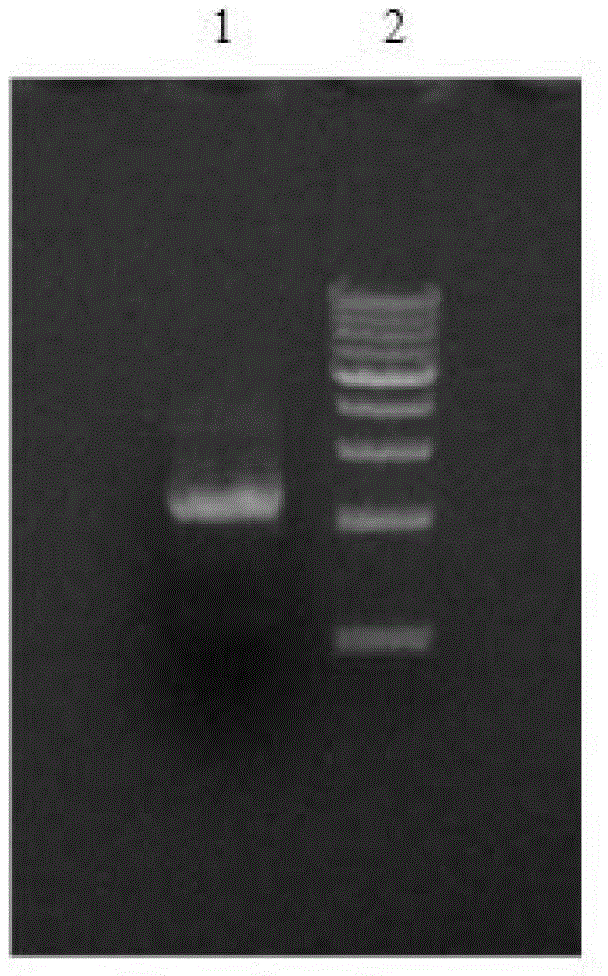
[0041] Digest pBS-CYP3A5-2A-POR with BamH I and Not I (results in Figure 9 ), insert the CYP3A5-2A-POR fragmen...
Embodiment 2
[0044] Example 2 Expression of Human Cytochrome P450 3A5 Enzyme in Pichia pastoris GS115
[0045] Positive clones were obtained after resistance and PCR screening, and the positive clones were subjected to methanol-induced expression for a certain period of time, and then the yeast cells were sonicated, and the protein expression was analyzed by conventional SDS-PAGE electrophoresis and Western Blot (see Figure 10 , 11 , 12), the results preliminarily proved that CYP3A5 was expressed in Pichia pastoris.
Embodiment 3
[0046] Example 3 Determination of Metabolic Midazolam Activity of Yeast Expressing Recombinant Human P4503A5 Enzyme
[0047] Homogenize the collected yeast cells with 0.1M phosphate buffer; centrifuge at 9,000g, 4°C for 20 minutes and at 100,000g, 4°C for 60 minutes to prepare microsomes by differential centrifugation; weigh in 0.1M phosphate buffer Suspended microsomes were precipitated, and the protein content was determined by the Bradford method, and the P450 content was determined by the carbon monoxide binding method; 0.45 mL of the mixture of the above-mentioned microsomes at an appropriate concentration and serially diluted midazolam was placed in a reaction tube, and kept in a 37°C water bath for 5 minute. Add 40 μL of 10 mM NADPH solution to start the reaction. After 6 minutes of reaction, take 0.1 mL of the reaction termination solution containing internal standard to terminate the reaction, mix well and place in ice water; centrifuge each of the above reaction tub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com