Cefixime composition and preparation method thereof
A technology of cefixime and cefixime oral preparations, which is applied in the field of cefixime composition and its preparation, can solve the problems of organic solvent residue, uneven content, and reduced dissolution rate, and achieve high mixing uniformity and particle size Uniform, stability-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
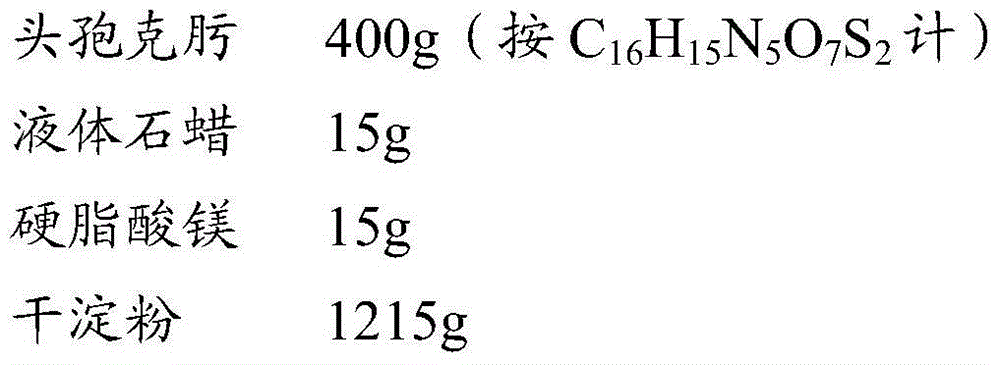
[0022] The preparation of embodiment 1 cefixime composition
[0023] Take the components:
[0024]
[0025] The above-mentioned 15g of liquid paraffin and 15g of magnesium stearate were mixed evenly to make it semi-solid, and 200g of cefixime was added and mixed through a 40-mesh sieve for 3 times. Add 200g of cefixime and mix well. Add 1215g of dry starch and mix evenly to get final product.
Embodiment 2
[0026] The preparation of embodiment 2 cefixime composition
[0027] Take the components:
[0028]
[0029] The above-mentioned 20g of liquid paraffin and 10g of magnesium stearate were mixed evenly to make it semi-solid, and 180g of cefixime was added and mixed through a 40-mesh sieve for 3 times. Add 180g of cefixime and mix well. Add 1500g of dry starch and mix evenly to get ready.
Embodiment 3
[0030] The preparation of embodiment 3 cefixime composition
[0031] Take the components:
[0032]
[0033] The above-mentioned 10g of liquid paraffin and 50g of magnesium stearate were mixed evenly to make it semi-solid, and 220g of cefixime was added and mixed through a 40-mesh sieve for 3 times. Add 220g of cefixime and mix well. Add 1100g of dry starch and mix evenly to get ready.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com