Method for preparing DL-isoserine
A technology of isoserine and acrylic acid, which is applied in the field of preparation of chiral organic compounds, can solve the problem of high cost, and achieve the effects of low environmental pollution, cheap and easy-to-obtain reaction raw materials, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
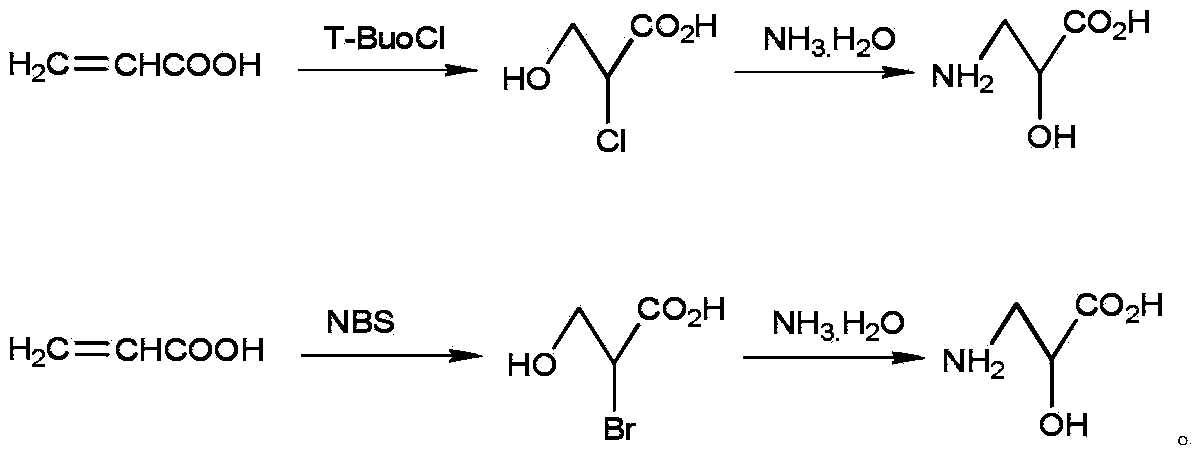
[0016] Add 5mL (0.077mol) of acrylic acid and 10mL of distilled water into a 100mL three-necked flask equipped with a magnetic stirrer. mL (0.077mol) of tert-butyl hypochlorite, after the dropwise addition, was concentrated in vacuo at 40°C to obtain 4.5 mL of oily liquid α-chloro-β-hydroxypropionic acid. Add 50mL of concentrated ammonia water to a 250mL three-necked flask equipped with a magnetic stirrer, and slowly add the above oily liquid dropwise under the condition of an ice-salt bath. Reflux for 1 h, filter, dry, recrystallize with water and ethanol, filter, and dry to obtain 5.66 g of DL-isoserine as a white powder, m.p.
example 2
[0018] Add 50mL (0.77mol) of acrylic acid and 100mL of distilled water into a 100mL three-neck flask equipped with a magnetic stirrer. The volume ratio is: acrylic acid: water = 1:2. Use an ice-salt bath to control the reaction temperature at about 0°C, and slowly add 92mL of (0.77mol) tert-butyl hypochlorite, after the dropwise addition, concentrated in vacuo at 40°C to obtain 4 mL of oily liquid α-chloro-β-hydroxypropionic acid. Add 500mL of concentrated ammonia water into a 1L three-necked flask equipped with a magnetic stirrer, and slowly add the above oily liquid dropwise under the condition of an ice-salt bath. Reflux for 1 hour, filter, dry, recrystallize with water and ethanol, filter, and dry to obtain 52.6 g of DL-isoserine as white powder, m.p.242-244°C, yield 65%.
example 3
[0020] Add 5mL (0.077mol) of acrylic acid and 60mL of distilled water into a 100mL three-necked flask equipped with a magnetic stirrer, and add 6.85g of NBS in batches at 20°C. After the reaction is complete, extract the reaction solution with ether three times, combine the extracts, and vacuum Ether was removed to obtain 4.5 mL of oily α-bromo-β-hydroxypropionic acid. Ammonolysis of the obtained α-bromo-β-hydroxypropionic acid is performed with the ammonolysis part of Example 1. The obtained crude product was refluxed with absolute ethanol, recrystallized with water and ethanol, and decolorized with activated carbon, filtered, left to stand overnight in the refrigerator, filtered, and dried to obtain 5.5 g of DL-isoserine in white powder form, with a total yield of 68 % (based on starting material acrylic acid).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com