Fluorescent detection reagent and detection method for dichlorinated-1, 1'-dimethyl-4, 4'-dipyridyl
A fluorescence detection and dimethyl technology, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., can solve the problems of cumbersome operation and difficulty in obtaining detection substances, and achieve high sensitivity, strong practical value, and safe use Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
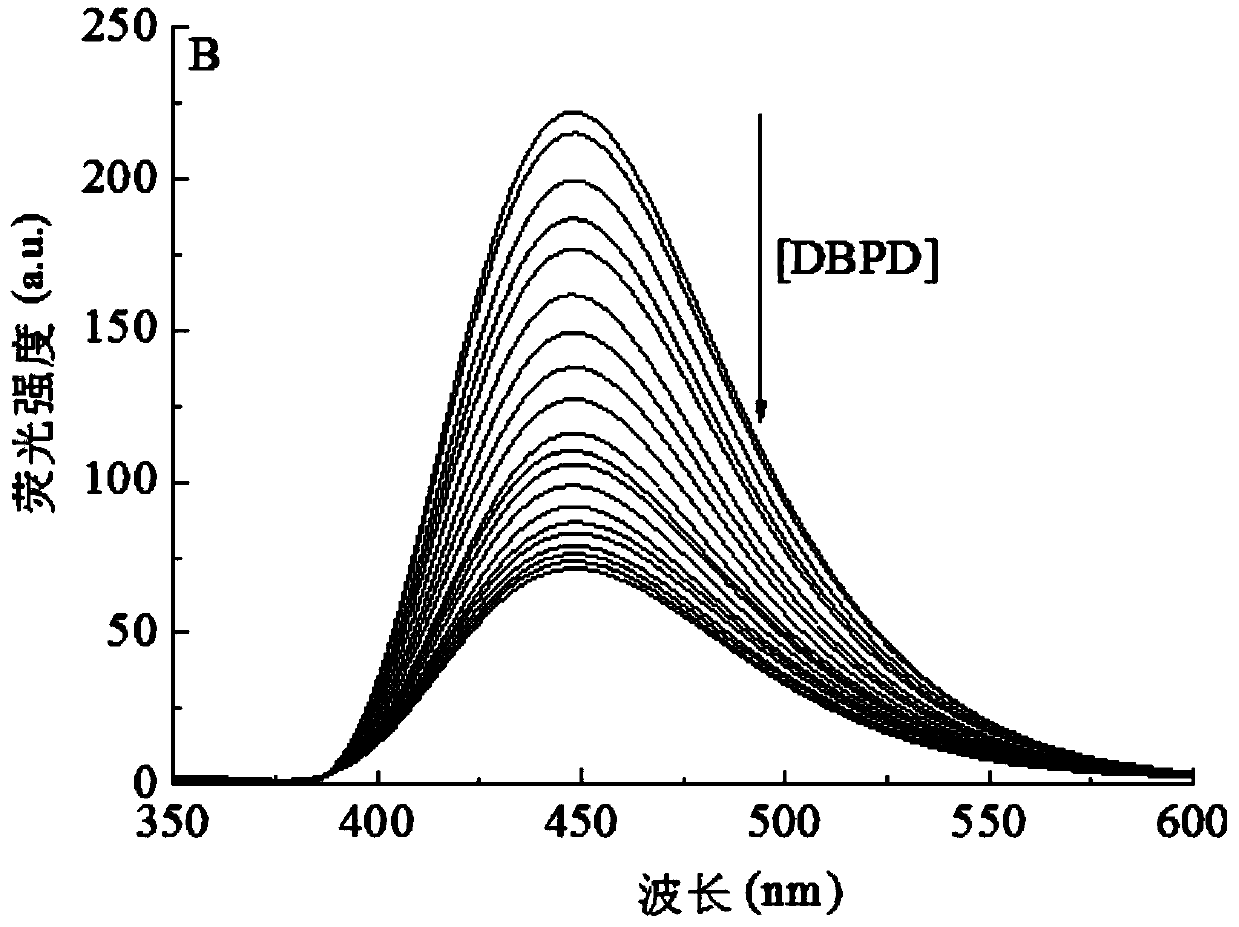
[0030] Specific Embodiment 1: A fluorescent detection reagent for 1,1'-dimethyl-4,4'-bipyridine dichloride in this embodiment uses the fluorescent dye CXT as the fluorescent molecular probe.
[0031] The fluorescent dye CXT described in this embodiment has a chemical name of 4,4'-bis[(4-hydroxyethylamino-6-anilino-1,3,5-triazin-2-yl)amino] -Sodium stilbene-2,2'-disulfonate, the molecular structure of which is shown in Formula 1 below.
[0032]
specific Embodiment approach 2
[0033] Specific embodiment two: The fluorescence detection reagent of dichloro-1,1'-dimethyl-4,4'-bipyridyl in this embodiment is composed of fluorescent dye CXT and a buffer solution, wherein the buffer solution is water It is prepared from a solvent, and the pH value of the buffer solution is 5-7.
specific Embodiment approach 3
[0034] Specific embodiment three: the difference between this embodiment and specific embodiment two is that the buffer solution is acetic acid-sodium acetate buffer solution, disodium hydrogen phosphate-citric acid buffer solution, disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution or hydrogen phosphate Dipotassium-sodium hydroxide buffer solution. Others are the same as in the second embodiment.
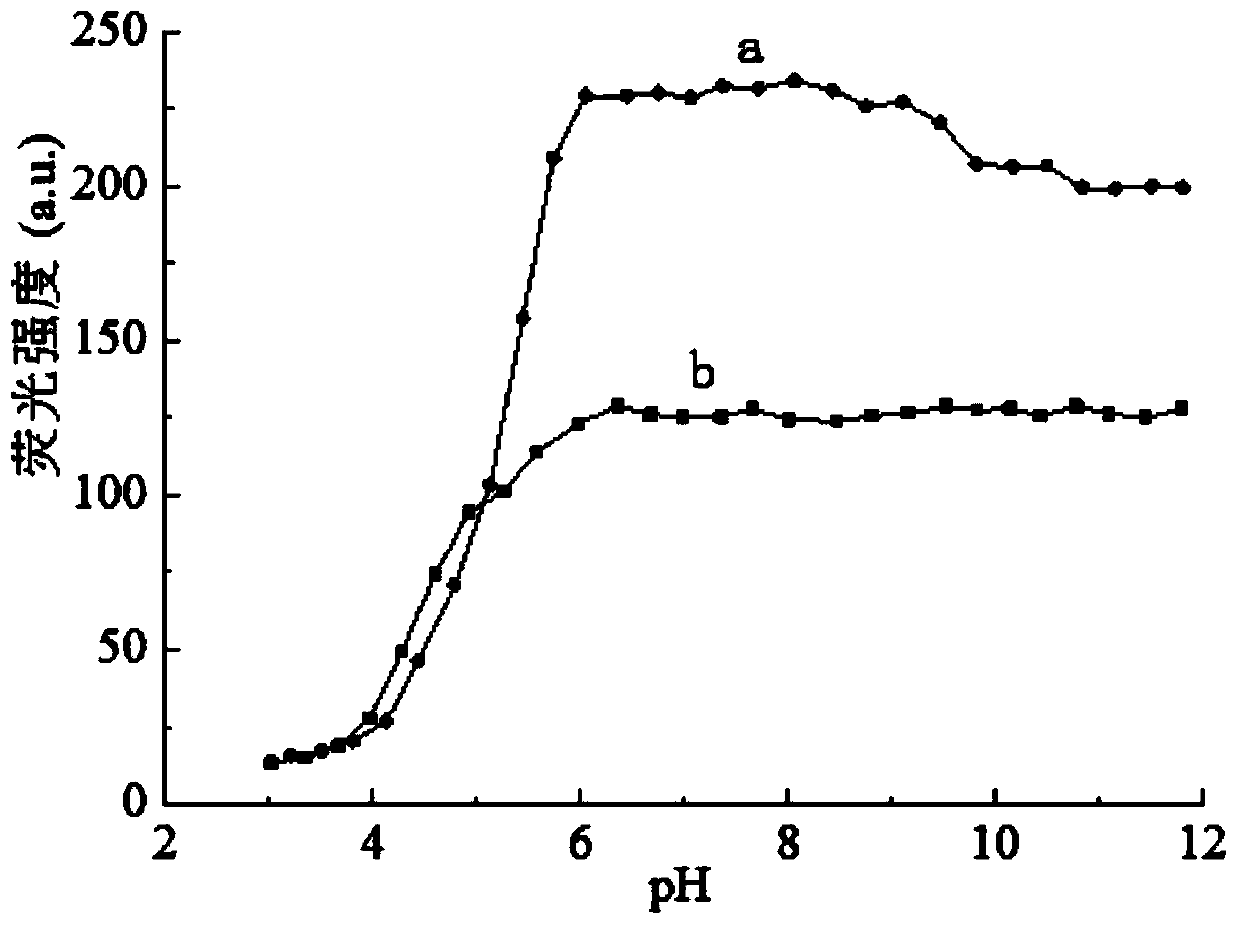
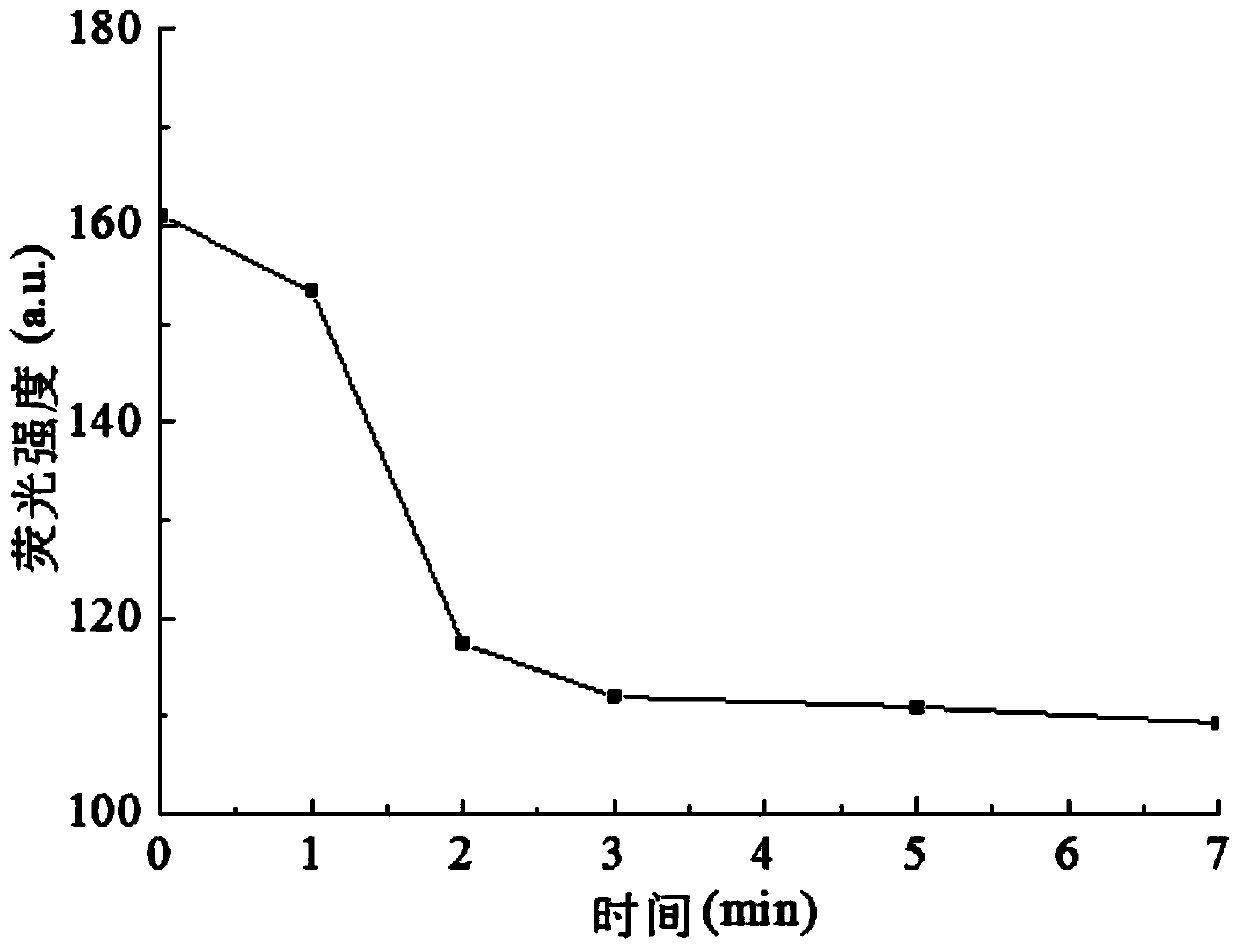
[0035] Since the method needs to be carried out in a weakly acidic solution, the pH of the solution needs to be controlled, so a buffer solution is needed to stabilize the pH value and ensure the stability of the pH value of the solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com