Method for secondary acid leaching of alumina in coal ash residues
A technology for coal ash slag and acid leaching slag, which is applied in the directions of silicate, alkali metal silicate, process efficiency improvement, etc. , low coal ash activity, etc., to achieve the effect of high labor productivity, advanced technology, and low pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
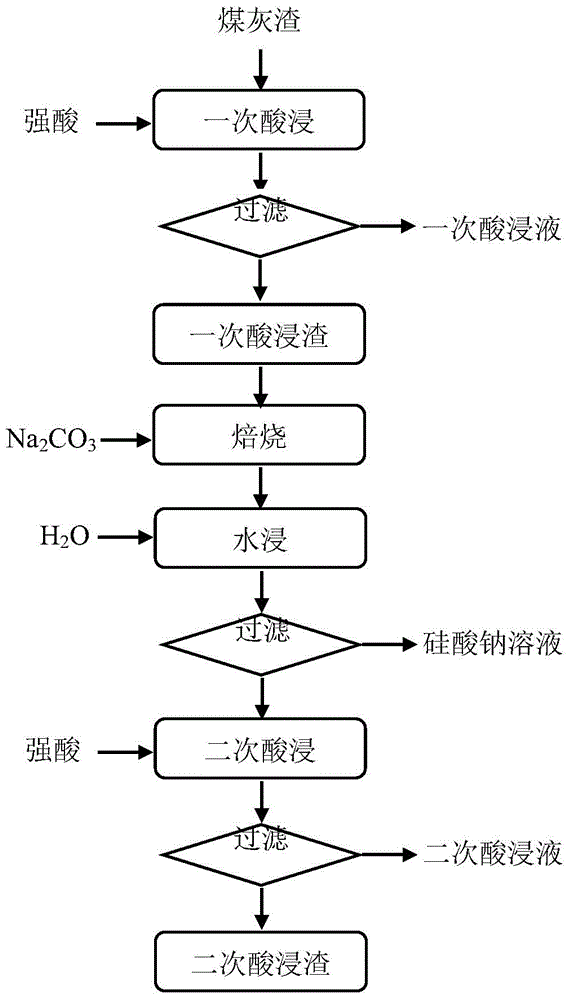
[0027] The aluminum-containing coal ash used is the bottom slag discharged after combustion in a circulating fluidized bed, and its chemical composition (mass fraction) is: SiO 2 59.38%, Al 2 o 3 27.34%, Fe 2 o 3 6.71%, CaO0.23%, MgO0.59%, K 2 O4.28%, Na 2 O0.58%, TiO 2 0.39%. Analysis of the occurrence state of aluminum in ash slag shows that part of aluminum exists in the form of amorphous state, and the other part of aluminum occurs in microplagioclase crystals.
[0028] The leaching steps of coal ash are as follows:
[0029] (1) One-time acid leaching: add 200mL of 10mol / L sulfuric acid solution into the flask, heat it to 120°C in an oil bath magnetic stirrer, add 100g of aluminum-containing lime ash, soak for 2 hours, filter and wash to obtain Aluminum-containing primary acid leaching solution and primary acid leaching residue;
[0030] (2) Roasting activation: the primary acid leaching residue contains SiO 2 83.14%, Al 2 o 3 8.33%, based on the molar ratio of...
Embodiment 2
[0035] The aluminum-containing coal ash used is the bottom slag discharged after combustion in the circulating fluidized bed, and its chemical composition is: SiO 2 59.38%, Al 2 o 3 27.34%, Fe 2 o 36.71%, CaO0.23%, MgO0.59%, K 2 O4.28%, Na 2 O0.58%, TiO 2 0.39%. Analysis of the occurrence state of aluminum in ash slag shows that part of aluminum exists in the form of amorphous state, and the other part of aluminum occurs in microplagioclase crystals.
[0036] The leaching steps of coal ash are as follows:
[0037] (1) One-time acid leaching: add 200mL of 10mol / L sulfuric acid solution in the flask, heat it to 140°C in an oil bath magnetic stirrer, add 100g of aluminum-containing lime ash, soak for 2 hours, filter and wash to obtain Aluminum-containing primary acid leaching solution and primary acid leaching residue;
[0038] (2) Roasting activation: the primary acid leaching residue contains SiO 2 84.31%, Al 2 o 3 8.12%, based on the molar ratio of anhydrous sodium...
Embodiment 3
[0043] The aluminum-containing coal ash used is the bottom slag discharged after combustion in the circulating fluidized bed, and its chemical composition is: SiO 2 59.38%, Al 2 o 3 27.34%, Fe 2 o 3 6.71%, CaO0.23%, MgO0.59%, K 2 O4.28%, Na 2 O0.58%, TiO 2 0.39%. Analysis of the occurrence state of aluminum in ash slag shows that part of aluminum exists in the form of amorphous state, and the other part of aluminum occurs in microplagioclase crystals.
[0044] The leaching steps of coal ash are as follows:
[0045] (5) One-time acid leaching: add 200mL of 10mol / L sulfuric acid solution into the flask, heat it to 120°C in an oil bath magnetic stirrer, add 100g of aluminum-containing lime ash, soak for 2 hours, filter and wash to obtain Aluminum-containing primary acid leaching solution and primary acid leaching residue;
[0046] (6) Roasting activation: the primary acid leaching residue contains SiO 2 83.14%, Al 2 o 3 8.33%, based on the molar ratio of anhydrous sod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com