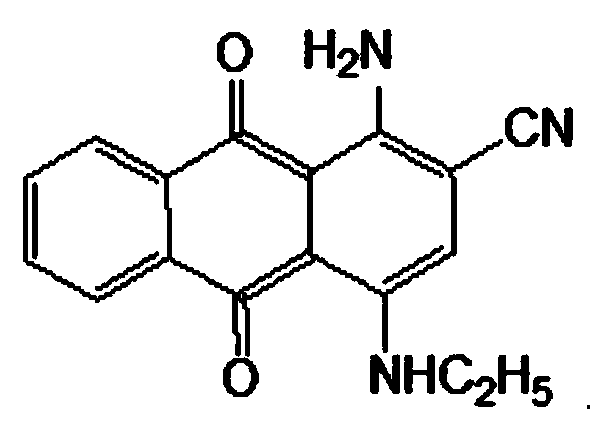
Disperse blue 359 preparation method
A technology of disperse blue and sulfonic acid, applied in chemical instruments and methods, organic dyes, anthracene dyes, etc., can solve problems such as difficult to stabilize product quality, low brilliance of disperse blue 359, great influence on yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Synthesis of Step 1 1-aminoanthraquinone-2-sulfonic acid:
[0023] In a 500ml four-neck flask, add 150g DMF and 25g 1-aminoanthraquinone, stir for 30 minutes, raise the temperature to 80°C, and add 14g chlorosulfonic acid dropwise at this temperature. After the dropwise addition was completed, the temperature was raised to 90° C., and the temperature was maintained for 8 hours until the end of the reaction. Cool down to below 70°C, and slowly add 800g of water dropwise. After cooling down to 5°C, the material was suction filtered, the filter cake was washed with hot water at 60°C until neutral, and the filter cake was dried. 34g of 1-aminoanthraquinone-2-sulfonic acid was obtained, the content was 98%, and the yield was 98%.
Embodiment 2
[0025] Synthesis of Step 1 1-aminoanthraquinone-2-sulfonic acid:
[0026] In a 500ml four-neck flask, add 200g DMF and 25g 1-aminoanthraquinone, stir for 30 minutes, raise the temperature to 80°C, and add 17.5g chlorosulfonic acid dropwise at this temperature. After the dropwise addition was completed, the temperature was raised to 95° C., and the temperature was maintained for 8 hours until the end of the reaction. Cool down to below 70°C, and slowly add 800g of water dropwise. After cooling down to 5°C, the material was suction filtered, the filter cake was washed with hot water at 60°C until neutral, and the filter cake was dried. 33.9g of 1-aminoanthraquinone-2-sulfonic acid was obtained, with a content of 98.2% and a yield of 97.9%.
Embodiment 3
[0028] Synthesis of Step 1 1-aminoanthraquinone-2-sulfonic acid:
[0029] In a 500ml four-neck flask, add 250g DMF and 25g 1-aminoanthraquinone, stir for 30 minutes, raise the temperature to 80°C, and add 20g chlorosulfonic acid dropwise at this temperature. After the dropwise addition, the temperature was raised to 100° C., and the temperature was maintained for 8 hours until the end of the reaction. Cool down to below 70°C, and slowly add 800g of water dropwise. After cooling down to 5°C, the material was suction filtered, the filter cake was washed with hot water at 60°C until neutral, and the filter cake was dried. 33.8 g of 1-aminoanthraquinone-2-sulfonic acid was obtained, with a content of 98.5% and a yield of 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com