Method for producing recombinant human BChE (butyrylcholinesterase) from transgenic animals by using gene knock-in and nuclear transfer technologies
A butyrylcholinesterase and genome sequence technology, applied in the field of biopharmaceuticals, can solve the problems of inhomogeneity of recombinant human butyrylcholinesterase products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0181] Example 1. Goat β-casein gene knock-in donor construct No. 1 construction
[0182] 1.1 Construction of the β-casein gene knock-in donor construct integrated in the goat β-casein gene intron 1:
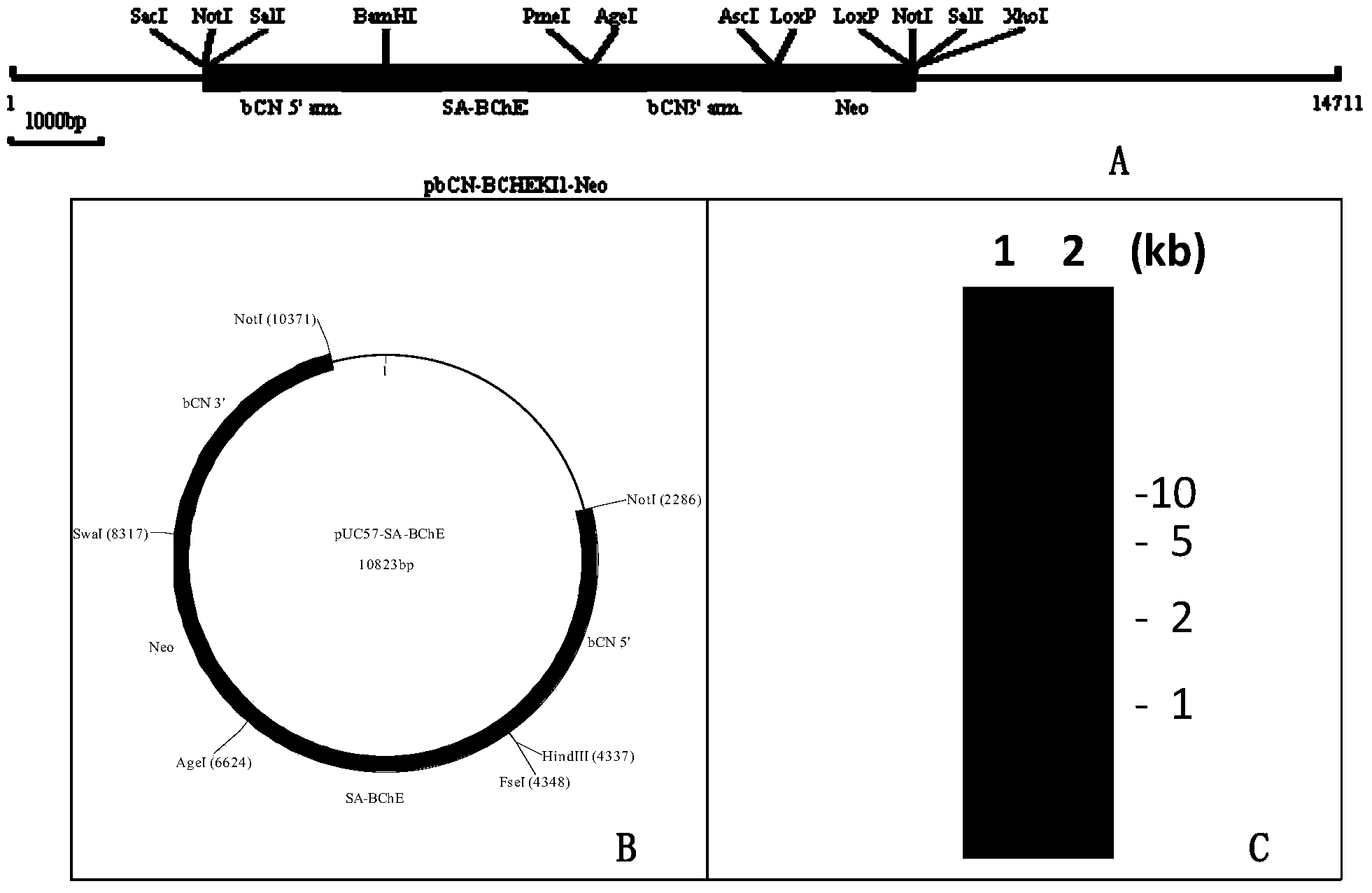
[0183] (i) Use a cis primer containing SacI, NotI, SalI sites and part of the 5'end homology arm sequence of the goat β-casein gene as shown in SEQ ID NO.: 7, and located in the goat β-casein promoter The trans primer containing the BamHI site at the midstream end of intron 1 is shown in SEQ ID NO.: 8. Using genomic DNA isolated from goat blood as a template, PCR amplification was performed to obtain a 2kb amplified product (5' homology arm sequence), the amplified product was then cloned into the pCDNA1-Neo plasmid (Invitrogen) by SacI-BamHI digestion to form pbCN5-Neo.
[0184] (ii) Use a cis primer containing BamHI site, goat β-casein signal sequence and part of human butyrylcholinesterase cDNA sequence as shown in SEQ ID NO.: 9, and containing XhoI, AscI, AgeI, PmeI The trans pri...
Embodiment 2
[0194] Example 2. Goat β-casein gene knock-in donor construct No. 2 construction
[0195] 2.1 Construction of a β-casein gene knock-in donor construct integrated into exon 8 of goat β-casein gene:
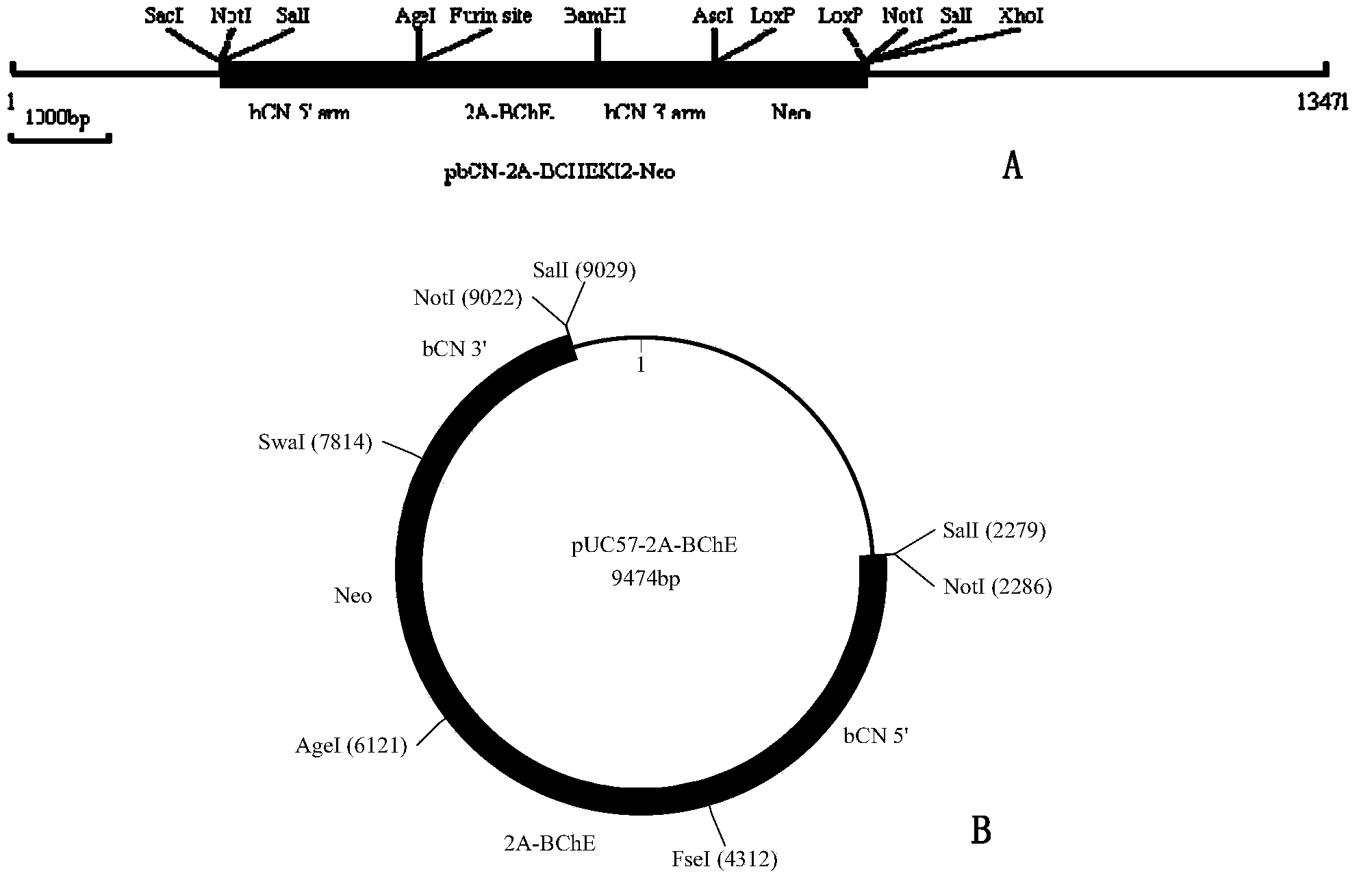
[0196] (i) Use a cis primer containing SacI, NotI, SalI sites and part of goat β-casein gene sequence as shown in SEQ ID NO.: 15 and containing AgeI site, part of 2A-polypeptide coding sequence and part of goat β -The trans primer of the casein gene sequence is shown in SEQ ID NO.: 16, using the genomic DNA isolated from goat blood as a template to perform PCR amplification to obtain a 2kb amplified product, which is then passed through SacI -AgeI digestion was cloned into pbCN-BChEKI1-Neo described in Example 1 to form pbCN52-Neo.
[0197] (ii) Use a cis primer containing AgeI site, part of 2A-polypeptide coding sequence, goat β-casein signal sequence and part of human butyrylcholinesterase cDNA sequence as shown in SEQ ID NO.: 17, and containing The trans primers of XhoI, AscI, BamHI ...
Embodiment 3
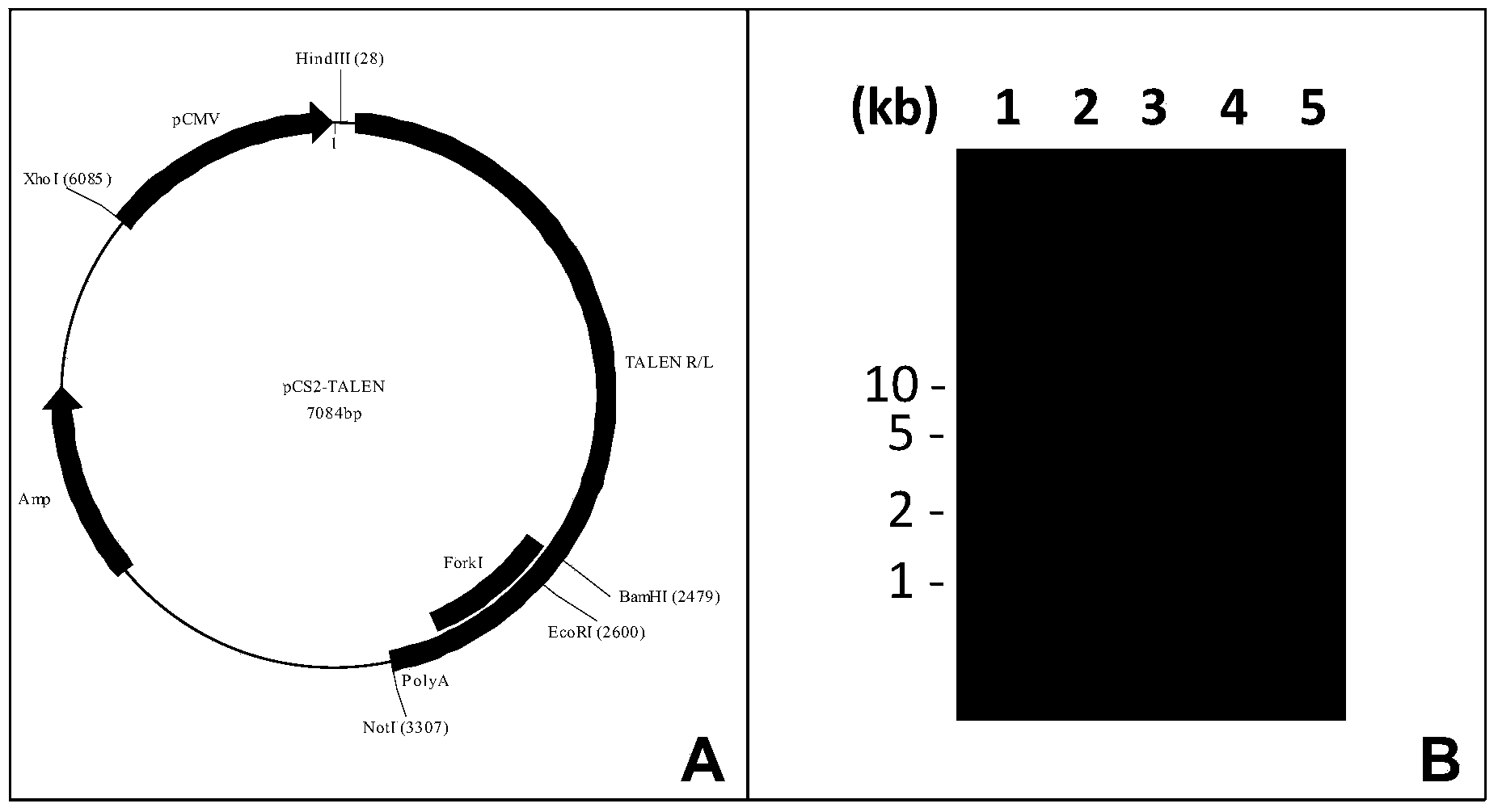
[0206] Example 3 TALEN vector construction:
[0207] Construct TALEN vector according to the method of gene synthesis ( image 3 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com