Protocatechuic acid-based liquid crystal compound with high fullerene content and preparation method thereof
A liquid crystal compound, protocatechuic acid technology, applied in the field of liquid crystal materials, can solve the problems of high phase transition temperature, limited application, low fullerene content, etc., and achieve high phase transition temperature, high space utilization, and orderly arrangement. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
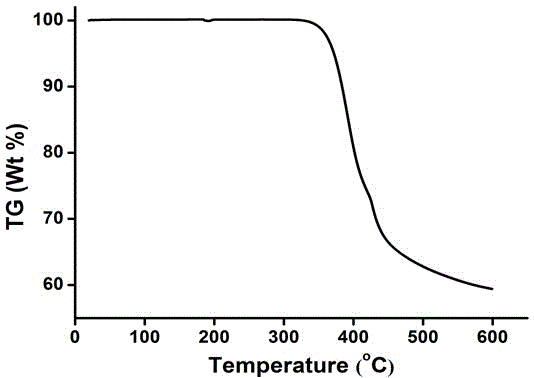
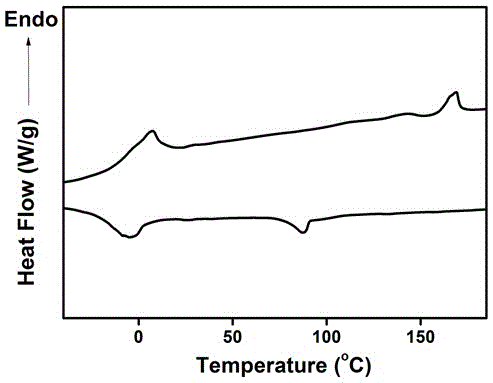
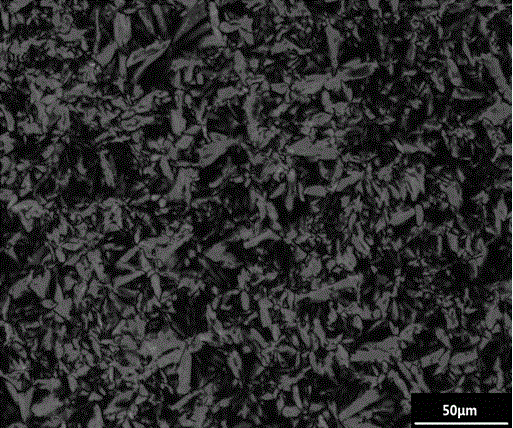
[0025] Because the synthetic route and the treatment mode of this series of compounds designed are similar, so further describe below in conjunction with accompanying drawing and embodiment:
[0026] 8-(3,4-bis(dodecyloxy)benzoate) octanediol 1-fullerene acetate (o-Bi-C 12 -C 8 -C 60 ) as an example:
[0027] Synthesis of Methyl 3,4-Di(dodecyloxy)benzoate
[0028]In a 250mL round-bottomed flask, add the reactant 3,4-dihydroxybenzoic acid methyl ester (5g), add the solvent DMF (50mL) to dissolve the reactant 3,4-dihydroxybenzoic acid methyl ester, add 1-bromododecanoate alkane (17g), add K 2 CO 3 (16.43g), reflux at 70oC for 24h under stirring, then pour the reaction solution into a mixed solution of toluene and deionized water (1:1), then separate the organic layer and the water layer, and wash the water layer with toluene 3 times, all the organic layers were collected, and the organic layer was washed to neutrality, dried with anhydrous sodium sulfate, left to stand o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com