Medicine elution balloon device
A drug and balloon technology, applied in balloon catheters, dilators, catheters, etc., can solve problems such as drugs that do not effectively prevent blood from scouring blood vessel walls.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
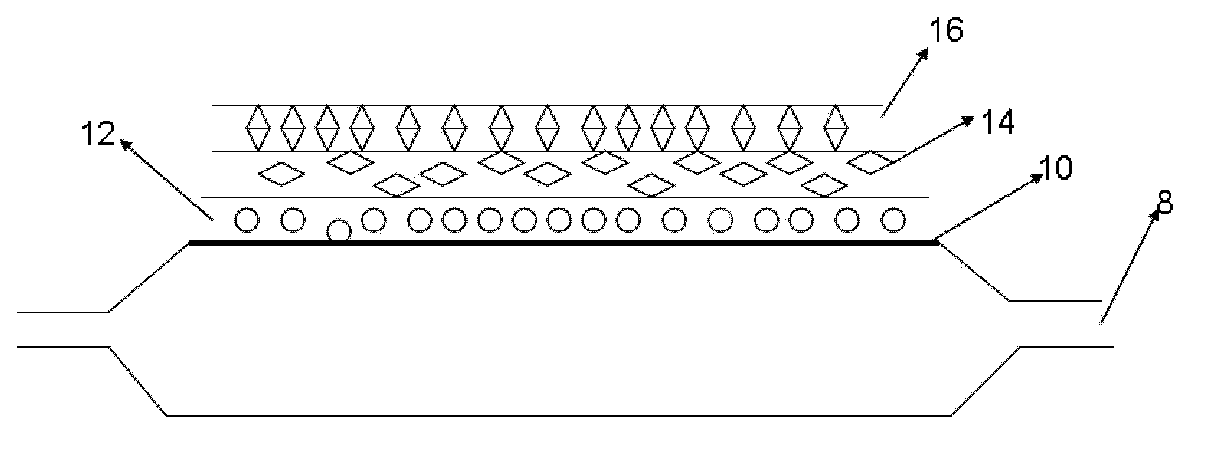
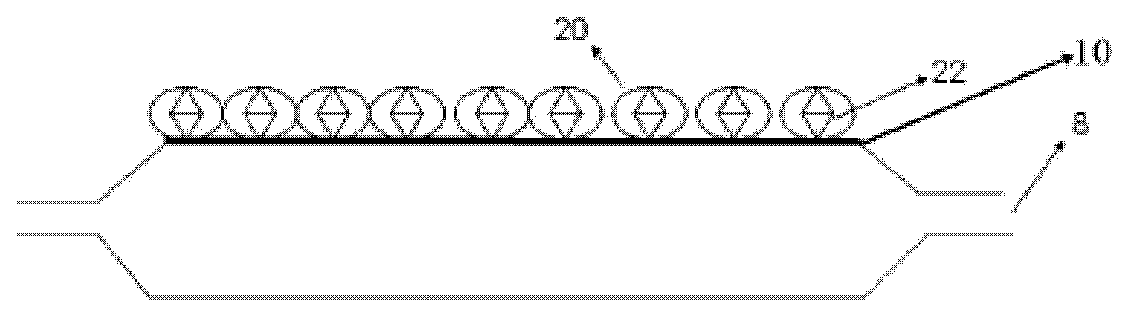
[0056] Take 5 balloons of 3.0*20 (material: Pebax), the balloon is the balloon 8 in the schematic diagram, the surface of the balloon is 10, scrub it with 75% ethanol, and set aside;
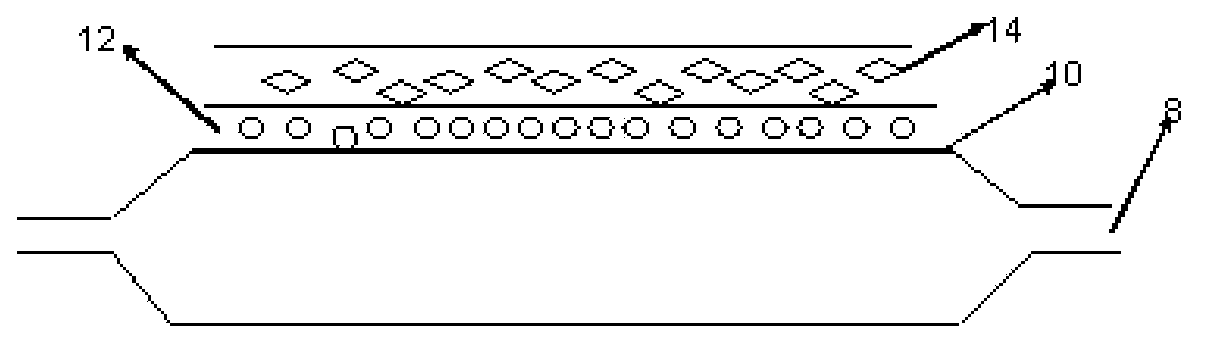
[0057] Dissolve 20mg of PVP (Wm=10000) in isopropanol to prepare a 25wt% solution, soak the above-mentioned balloon in the above-mentioned solution for 1 second, take it out, and dry it by ultraviolet curing to form a dissolvable and releasable bottom layer 12;
[0058] Dissolving 5 mg of PLGA in THF solution to form a solution with a concentration of 50 wt%, soaking the above-mentioned balloon in the solution for 3 seconds, taking it out, curing it with ultraviolet rays and drying it to form the polymer layer 14;
[0059] Soak the above-mentioned balloon again in pure water at 45°C for half an hour until the bottom layer 12 is completely dissolved and "extracted";
[0060] Dissolve 80mg of paclitaxel in 5:1 (volume ratio) acetone and ethanol solution, the prepared concentration is
[0061] 20m...
Embodiment 2
[0063] Take 5 3.0*20 balloons (material: nylon 12), the balloon is the balloon 8 in the schematic diagram, the surface of the balloon is 10, scrub with 75% ethanol, and set aside;
[0064] Dissolve 1g of sodium alginate in water to form a solution with a concentration of 1wt%, immerse the above-mentioned balloon in the above-mentioned solution for 1 second, take it out, and immerse the balloon in 5wt% of CaCl 2 In aqueous solution, Ca 2+ with Na + For exchange, the balloon is taken out, rinsed with deionized water, and dried to form a calcium alginate layer 14;
[0065] Dissolve 80mg of paclitaxel in 5:1 (volume ratio) acetone and ethanol solution, the prepared concentration is
[0066] 20mg / ml solution, spray this solution on the surface of the above-mentioned balloon to form 1μg / mm 2 coated, dried, folded, and packaged, and the resulting balloon was called DEB2.
Embodiment 3
[0068] Take 5 3.0*20 balloons (material: nylon 12), the balloon is the balloon 8 in the schematic diagram, the surface of the balloon is 10, scrub with 75% ethanol, and set aside;
[0069] Dissolving 2mg of polyhexanediol and 0.2mg of propylene glycol in a mixed solution of 10ml of isopropanol and water to form a solution with a concentration of 10-80wt%;
[0070] 8 mg of polyhydroxybutyrate was dissolved in the above solution to form a latex solution;
[0071] Dissolve 40 mg of paclitaxel in the above solution to form a "core-shell" structure;
[0072] Soak the balloon in the above solution to form 3μg / mm 2 coated, dried, folded, and packaged, and the resulting balloon was called DEB3.
[0073] In order to verify the drug loss of the drug balloon during the delivery process, the above-mentioned balloon that was folded and pressed was delivered into the arteries of New Zealand white rabbits weighing about 2.5 kg for flushing. Measure the remaining drug residue rate on the b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com