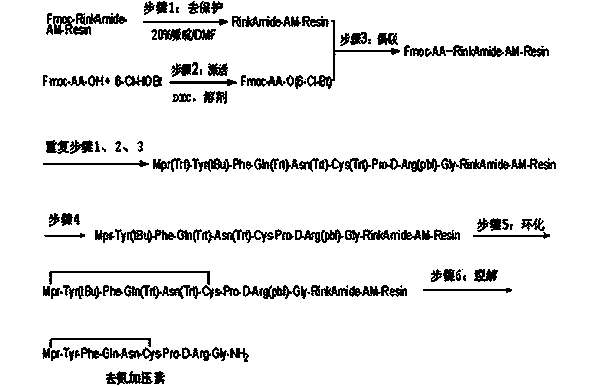
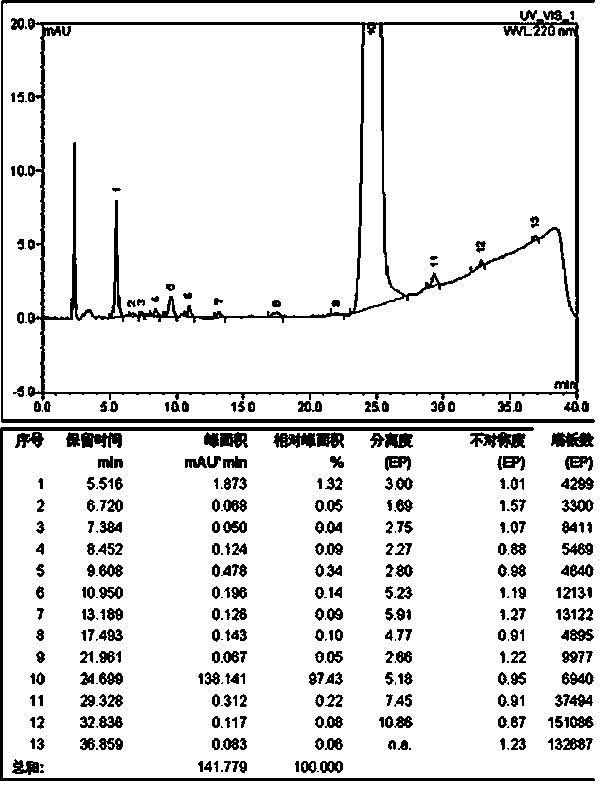
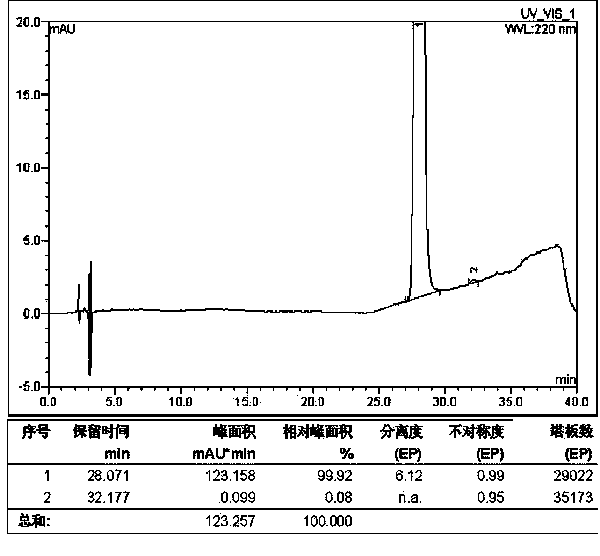
Method for solid cyclizing synthesis of desmopressin
A technology of desmopressin and preparation method, which is applied in the field of preparation of polypeptide drugs, and can solve the problems of low yield, low yield, and many by-products of solid-phase synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Preparation of oxidizing agent: 56.56g of urea peroxide was dissolved in 1500 mL of DMSO.
Embodiment 2
[0074] Step 1 Synthesis of Fmoc-Gly-RinkAmide-AM-Resin
[0075] Weigh Rink Amide AM resin (substitution degree 0.73mmol / g) (164.85 g, 1.0 equiv) into a solid phase reactor, add DCM (1000mL), stir and swell at a temperature of 10-30°C for 30min, and remove the liquid by suction filtration. Then add DMF (1000mL×2) to wash. Measure 20% (v / v, the same below) Piperidine / DMF solution (850 mL), add it into the solid-phase reactor, control the temperature at 20-30°C and stir for 5 minutes, then remove the liquid by suction filtration. Measure 20% Piperidine / DMF solution (850 mL) again, add it to the solid-phase reactor, control the temperature at 20-30°C and stir for 10 minutes, then remove the liquid by suction filtration. Measure DMF (1000 mL×6) into the solid-phase reactor for washing, and remove the liquid by suction filtration. Separately weigh Fmoc-Gly-OH (107.13 g, 3.0 equiv) and Cl-HOBt (67.15 g, 3.3 equiv) into the beaker. Measure DMF (450 mL) into a beaker, stir to dissol...
Embodiment 3
[0089] Step 1 Synthesis of Fmoc-Gly-RinkAmide-AM-Resin
[0090] Weigh Rink Amide AM resin (substitution degree 0.73mmol / g) (1648.5 g, 1.0 equiv) into a solid phase reactor, add DCM (10 L), stir and swell at a temperature of 10-30°C for 30 minutes, and remove the liquid by suction filtration. Then add DMF (10 L×2) to wash. Measure 20% (v / v, the same below) Piperidine / DMF solution (8.50 L), add it into the solid phase reactor, control the temperature at 20-30°C and stir for 5 minutes, then remove the liquid by suction filtration. Measure 20% Piperidine / DMF solution (8.50 L) again, add it into the solid phase reactor, control the temperature at 20-30°C and stir for 10 min, then remove the liquid by suction filtration. Measure DMF (10L×6) into the solid-phase reactor for washing, and then remove the liquid by suction filtration. Separately weigh Fmoc-Gly-OH (1071.3 g, 3.0 equiv) and Cl-HOBt (671.5 g, 3.3 equiv) into the dissolution kettle. Measure DMF (4.50 L) into the dissolut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com