Enclosed formaldehyde virus-inactivating pipeline system and applications thereof
A pipeline system and formaldehyde inactivation technology, which is applied in the field of closed formaldehyde inactivation virus pipeline system, can solve the problems of increasing the operation steps of technicians, increasing the degree of pollution risk, and the need for repeated assembly, so as to avoid external pollution and save energy. Production cost, the effect of ensuring sealing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Closed formaldehyde inactivation virus pipeline system
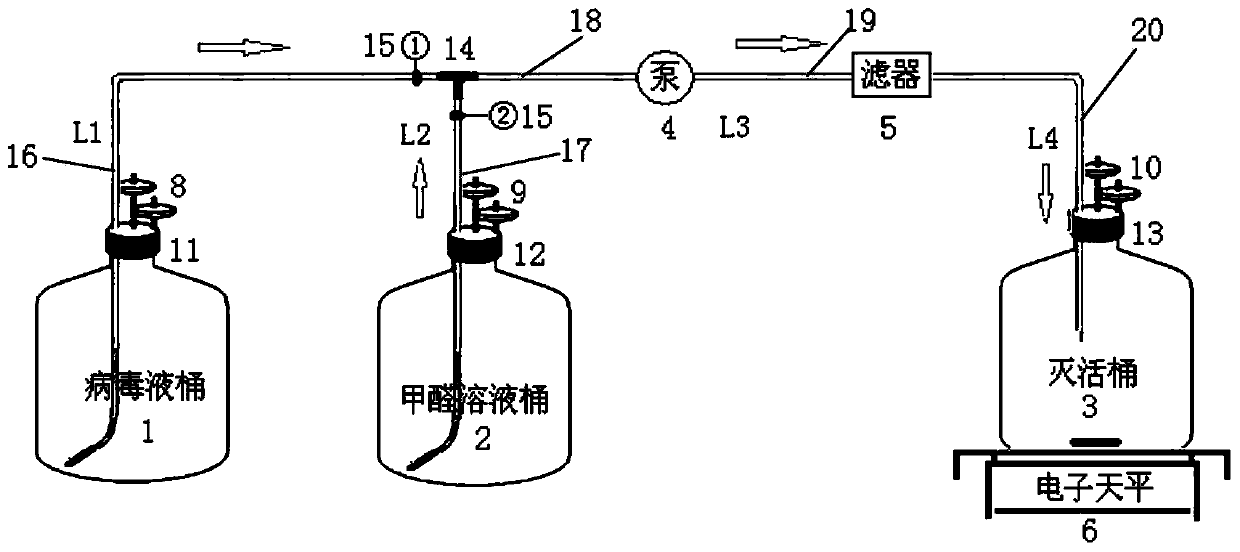
[0033] The closed formaldehyde inactivating virus pipeline system provided in this embodiment has a schematic structural diagram such as figure 1 with 2 Shown. The pipeline system includes virus liquid tank 1, pipeline L116, formaldehyde solution tank 2, pipeline L217, T tee 14, pipeline L318, peristaltic pump 4, pipeline L419, sterilizing filter 5, pipeline L520, inactivation tank 3, Balance 6 and magnetic stirrer 7;
[0034] The pipe L116 extends into the barrel through the lid 11 of the virus liquid barrel to ensure that one side of the pipe L1 is in contact with the barrel bottom. The lid 11 is connected with an air filter 8, and the other end of the pipe L116 passes through the pipe L217 and the pipe L318. Tee 14-phase connection;
[0035] The pipe L217 extends into the barrel through the lid 12 of the formaldehyde solution barrel to ensure that one side port of the pipe L2 is in contact with the bottom ...
Embodiment 2
[0047] Example 2 Using closed formaldehyde inactivation virus pipeline system to inactivate hepatitis A virus liquid
[0048] The hepatitis A virus liquid was inactivated by using the formaldehyde inactivation virus pipeline system in Example 1. The details of the intermediate control and aseptic production operation methods during the inactivation operation are as follows:
[0049] 1. After culturing human diploid cells in a cell factory, inoculating hepatitis A virus, add cell lysate to harvest hepatitis A virus solution.
[0050] 2. The hepatitis A virus harvest liquid is purified and refined to obtain a relatively pure hepatitis A virus liquid.
[0051] 3. Inactivate hepatitis A virus liquid to produce inactivated hepatitis A virus liquid.
[0052] (1) After cleaning and disinfecting the inactivation room, connect the combined pipeline.
[0053] (2) Hepatitis A virus liquid filtration ( figure 1 ): Connect virus liquid tank 1 figure 1 Middle L1 pipeline, and 15 hemostatic forceps fi...
Embodiment 3
[0056] Example 3 Use of closed formaldehyde inactivation virus pipeline system to inactivate polio virus liquid
[0057] The polio virus liquid was inactivated using the formaldehyde inactivation virus pipeline system in Example 1. The details of the intermediate control and aseptic production operation methods during the inactivation operation are as follows:
[0058] 1. After resuscitation and expansion of human diploid cells, the polio virus is harvested after inoculation with polio virus seeds.
[0059] 2. The polio virus harvest liquid is purified and refined to obtain a relatively pure polio virus liquid.
[0060] 3. Inactivate polio virus liquid to produce inactivated polio virus liquid.
[0061] (1) After cleaning and disinfecting the inactivation room, connect the combined pipeline.
[0062] (2) Polio virus fluid filtration ( figure 1 ): Connect virus liquid tank 1 figure 1 Middle L1 pipeline, and 15 hemostatic forceps figure 1 The middle point ② is clamped at the pipe, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com