Method for regenerating catalysts for olefin esterification reaction and hydration reaction
A technology of hydration reaction and catalyst, which is applied in the chemical industry to achieve the effect of simple regeneration process, easy recovery and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Weigh 20g of the CT800 deactivated catalyst, weigh 50g of acetic acid, pour it into a 150ml beaker, control the solvent immersion temperature at 35°C, and the immersion time for 60h. The solution was poured into a Buchner funnel, the catalyst was separated by suction filtration, and the solvent was recovered for reuse next time. The catalyst was washed with distilled water until the pH of the washing water was neutral. Put the catalyst into a watch glass, put the watch glass into an oven, the drying temperature is 100°C, and the drying time is 24 hours.
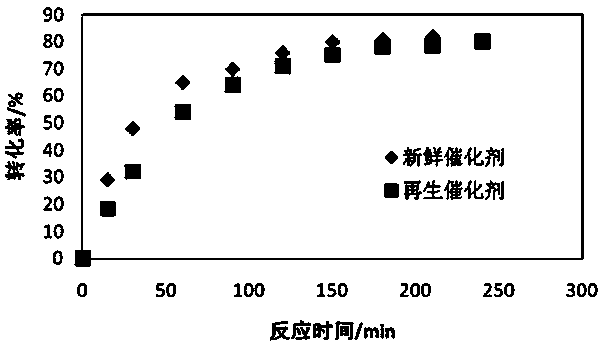
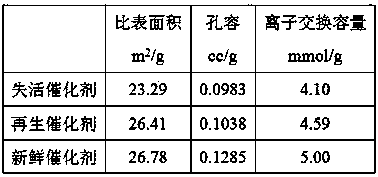
[0025] In a batch reactor, under the same reaction conditions (reaction temperature 40°C, stirring speed 400rpm, molar ratio of alkene to acid 1:1.1, catalyst dosage 10% of the mass of the reaction liquid), the catalyst was studied in the esterification of camphene to prepare acetic acid iso Catalytic activity in the borneol ester reaction, the regenerated CT800 catalyst was compared with the fresh catalyst, and the r...
Embodiment 2
[0030] Weigh 20g of the NKC-9 deactivated catalyst, weigh 60g of ethanol, pour it into a 150ml beaker, control the solvent immersion temperature at 25°C, and the immersion time for 48h. The solution was poured into a Buchner funnel, the catalyst was separated by suction filtration, and the solvent was recovered for reuse next time. The catalyst was washed with distilled water until the pH of the washing water was neutral. Put the catalyst into a watch glass, put the watch glass into an oven, the drying temperature is 85°C, and the drying time is 36 hours.
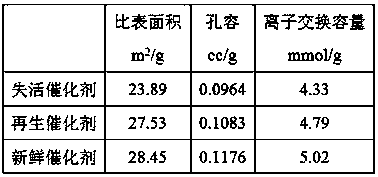
[0031] The specific surface area, pore volume and ion exchange capacity of the regenerated NKC-9 catalyst were measured, and the measured values were close to those of the new catalyst, as shown in Table 2.
[0032] Table 2 The specific surface area, pore volume and ion exchange capacity of the catalyst
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com