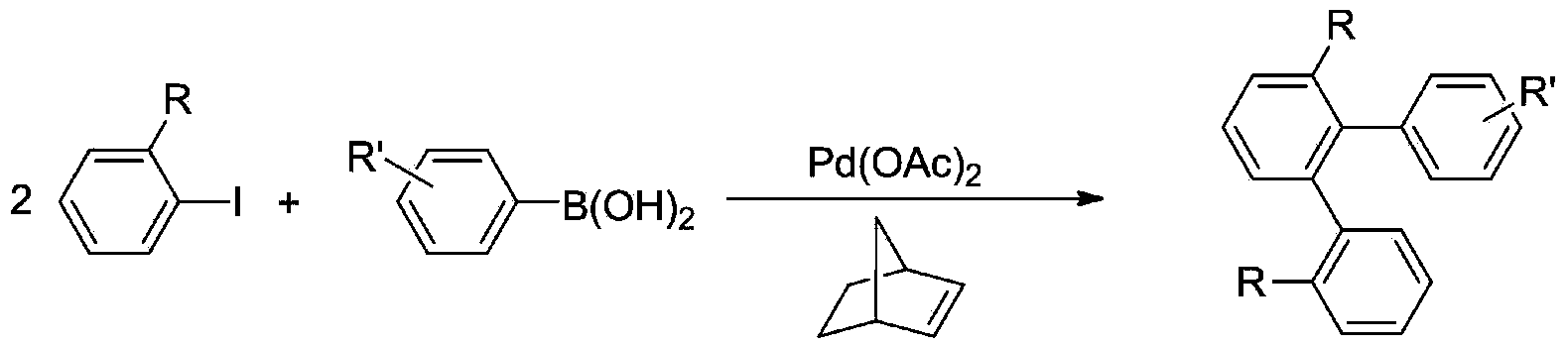
Method for synthetizing terphenyl compounds through palladium catalyzed cascade reaction
A series reaction, terphenyl technology, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of environmental pollution, high catalyst dosage, high acid dosage, etc., and achieve high application value and product yield. High, easy post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The synthesis of embodiment 1 2'-acetamido terphenyl
[0034] Add 3-bromoacetanilide (0.2mmol, 42.81mg), palladium acetate (0.002mmol, 0.45mg), iodobenzene (0.22mmol), K 3 PO 4(0.6mmol, 127.36mg), silver acetate (0.3mmol, 50.07mg), TFA (0.1mmol) and DCE (2mL), reacted at 90 ℃, TLC monitored the completion of the reaction, when the temperature dropped to room temperature, added benzene Boric acid (1.2mmol, 29.26mg) reacted with the ligand diphenol (0.004mmol, 1.07mg) at 80°C, and the reaction was detected by TLC. When the reaction is down to room temperature, add 5 mL of diethyl ether, pour the reaction solution into a separatory funnel, rinse the reaction bottle with 5 mL of diethyl ether, then merge the diethyl ether into the separatory funnel, add 10 mL of saturated NaCl solution to the separatory funnel, shake, and divide liquid, and then extracted with 10mL×2 diethyl ether, combined the organic phases, added anhydrous sodium sulfate to dry, filtered, and the solve...
Embodiment 2
[0037] Example 2 Synthesis of 4-methyl-2'-acetamido terphenyl
[0038] Add 3-bromoacetanilide (0.2mmol, 42.81mg), palladium acetate (0.002mmol, 0.45mg), 4-methyliodobenzene (0.22mmol, 47.97mg), K 3 PO 4 (0.6mmol, 127.36mg), silver acetate (0.3mmol, 50.07mg), TFA (0.1mmol) and DCE (2mL), reacted at 90 ℃, TLC monitored the completion of the reaction, when the temperature dropped to room temperature, added benzene Boric acid (1.2mmol, 29.26mg) and ligand diphenol (0.004mmol, 1.07mg) continued to react at 80°C, and the end point of the reaction was detected by TLC. When the reaction is down to room temperature, add 5 mL of diethyl ether, pour the reaction solution into a separatory funnel, rinse the reaction bottle with 5 mL of diethyl ether, then merge the diethyl ether into the separatory funnel, add 10 mL of saturated NaCl solution to the separatory funnel, shake, and divide liquid, and then extracted with 10mL×2 diethyl ether, combined the organic phases, added anhydrous sod...
Embodiment 3
[0041] Example 3 Synthesis of 4-chloro-2'-acetamido terphenyl
[0042] Add 3-bromoacetanilide (0.2mmol, 42.81mg), palladium acetate (0.002mmol, 0.45mg), 4-chloroiodobenzene (0.22mmol, 52.46mg), K 3 PO 4 (0.6mmol, 127.36mg), silver acetate (0.3mmol, 50.07mg), TFA (0.1mmol) and DCE (2mL), reacted at 90 ℃, TLC monitored the completion of the reaction, when the temperature dropped to room temperature, added benzene Boric acid (1.2mmol, 29.26mg) and ligand diphenol (0.004mmol, 1.07mg) continued to react at 80°C, and the end point of the reaction was detected by TLC. When the reaction is down to room temperature, add 5 mL of diethyl ether, pour the reaction solution into a separatory funnel, rinse the reaction bottle with 5 mL of diethyl ether, then merge the diethyl ether into the separatory funnel, add 10 mL of saturated NaCl solution to the separatory funnel, shake, and divide liquid, and then extracted with 10mL×2 diethyl ether, combined the organic phases, added anhydrous sod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com