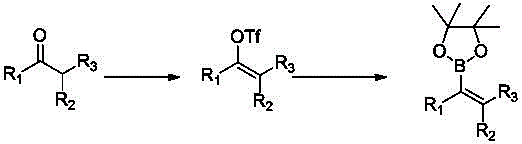
Synthetic method of 3,6-dihydro-2H-pyran-4-boronic acid pinacol ester
A technology of pinacol ester and synthesis method, which is applied in the fields of compounds, chemical instruments and methods, and organic chemistry containing elements of Group 3/13 of the periodic table, and can solve the problems of inflammability and explosion, high production cost and high production cost. and other problems, to achieve the effects of reducing production costs, simple synthesis methods, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] A kind of synthetic method of 3,6-dihydro-2H-pyran-4-boronic acid pinacol ester, comprising steps:
[0030] 1) Tetrahydropyrone reacts with hydrazine hydrate in a solvent to form a monoketone hydrazone;
[0031] 2) Monoketone hydrazone and copper bromide react in a solvent in the presence of triethylamine to obtain a dibromo intermediate;
[0032] 3) The dibromo intermediate reacts with the base in a solvent to obtain an alkenyl bromide compound;
[0033] 4) In the presence of palladium complexes, alkenyl bromide compounds and biboronic acid pinacol esters undergo a Suzuki-Miyaura reaction in a solvent to obtain the product.
[0034] In step 1), the molar ratio of tetrahydropyrone to hydrazine hydrate is 1: (1-20); preferably, it is 1: (2-5).
[0035] In step 1), the solvent is dichloromethane, dichloroethane, trichloroethane, tetrachloroethane, diphenyl ether, diethyl ether, methyl tert-butyl ether, ethylene glycol dimethyl ether, ethyl ether, One of glycol diethyl ...
Embodiment 1
[0047] Synthesis of tetrahydropyrone hydrazone
[0048] Under stirring, 5.0 L of dichloromethane, 2.5 kg of 80% hydrazine hydrate and 1.0 kg of tetrahydropyrone were added dropwise to the reaction kettle at 20-30°C. After the dropwise addition was complete, stirring was continued at this temperature for 2 hours. 2.0 L of saturated brine was added, the layers were allowed to stand, and the organic phase was concentrated to obtain 830 g of tetrahydropyrone hydrazone, with a yield of 73%.
Embodiment 2
[0050] Synthesis of 4,4-Dibromotetrahydropyran
[0051] Under the protection of nitrogen and at room temperature, 5.0 L of methanol, 12.0 kg of copper bromide and 7.5 L of triethylamine were added to the reaction kettle. After the addition was complete, the stirring was continued at room temperature for half an hour. A methanol solution containing 1.0 kg of tetrahydropyrone hydrazone (the volume of the solution is 2 L) was added dropwise into the reaction kettle, and stirring was continued for half an hour at room temperature. Add 15 L of ammonia water and 10 L of ethyl acetate, let stand for separation, and concentrate the organic phase to obtain the crude product, which is decolorized by activated carbon to obtain 1.7 kg of 4,4-dibromotetrahydropyran (purity greater than 95%), with a yield of 80 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com