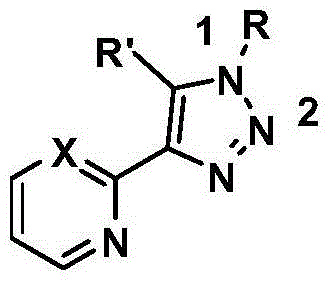
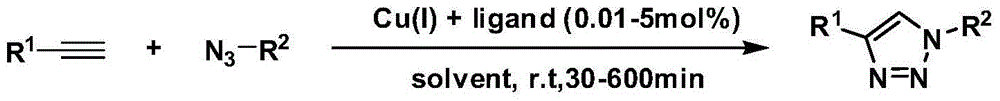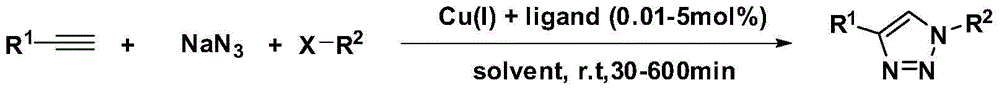N1 substituted 1,2,3-triazole derivative for ligand of Cu(I) as well as preparation method and application of N1 substituted 1,2,3-triazole derivative
A technology of triazole derivatives and ligands, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, chemical/physical processes, etc., can solve the problem of low reaction yield, difficult synthesis, easy salt Disproportionation and other problems, to achieve the effect of high product yield, strong substrate adaptability and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Synthesis of Ligand 1
[0029]
[0030] In a 250mL round bottom flask, add pyridylacetylene (14.5g, 100mmol), benzyl azide (15.7g, 110mmol), CuSO 4 ·5H 2 O (1.25g, 5mmol), sodium ascorbate (1.98g, 10mmol), using anhydrous methanol (180mL) as the reaction solvent, stirred at room temperature for 30min to 1h to complete the reaction. After the reaction, use a rotary evaporator to remove absolute ethanol, concentrate to obtain a crude product, and separate and purify by thin-layer silica gel column chromatography to obtain a white solid, 12.3 g, with a yield of 52%. The structure of the compound is characterized as follows: 1H NMR (400MHz, CDCl3) δ8.64(s, 1H), 8.18(s, 1H), 7.91(d, J=7.2Hz, 1H), 7.74(t, J=7.2Hz ,1H),7.35-7.22(m,6H),5.65(s,2H).
Embodiment 2
[0032] Synthesis of Ligand 2
[0033]
[0034] In a 250mL round bottom flask, add pyridylacetylene (14.5g, 100mmol), 2-pyridine azido (17.4g, 110mmol), CuSO 4 ·5H 2 O (1.25g, 5mmol), sodium ascorbate (1.98g, 10mmol), using anhydrous methanol (180mL) as the reaction solvent, stirred at room temperature for 30min to 1h to complete the reaction. After the reaction, the absolute ethanol was removed by a rotary evaporator, concentrated to obtain a crude product, separated and purified by thin-layer silica gel column chromatography to obtain a white solid, 13.5 g, with a yield of 61%. The structure of the compound is characterized as follows: 1H NMR (400MHz, CDCl3) δ9.18 (s, 1H), 8.66 (d, J = 4.4Hz, 1H), 8.55 (d, J = 4.4Hz, 1H), 8.26-8.23 (m,2H),7.94(t,J=7.6Hz,1H),7.82(t,J=7.6Hz,1H),7.40-7.35(m,1H),7.29(d,J=8.8Hz,1H) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com