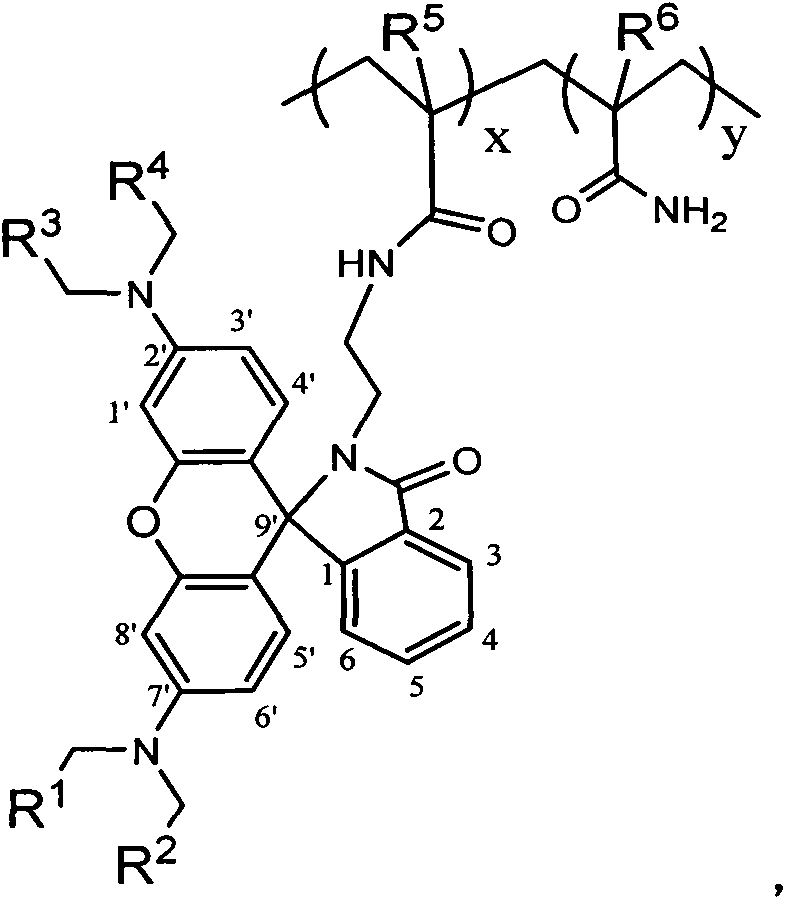
High-molecular pH probe containing rhodamine lactam group and synthetic method thereof
A technology of clearing lactam and polymer, applied in the field of polymer pH probe and synthesis, can solve the problems of hydrogen ion fluorescence response interference and other problems, and achieve the effect of good anti-interference ability and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Synthesis of compound 1:
[0026] Dissolve 2.395g of rhodamine B (5mmol) in 50mL of absolute ethanol, and then add 1.5g of ethylenediamine. The reaction system was stirred at reflux for 6 hours. After the reaction was completed, the solvent was evaporated under reduced pressure, and the remaining solid was dissolved in 200mL of 1mol / L HCl solution, and 1mol / L NaOH solution was added dropwise to pH=9-10. A large amount of solid precipitated, filtered under reduced pressure, washed with water, and then thoroughly pumped. After drying in a vacuum oven, a brick-red solid was obtained, namely compound 1.
[0027] (2) Synthesis of compound 2:
[0028] Add 0.968g of compound 1 (2mmol) into a 50mL three-necked flask, dissolve it in 20mL of dichloromethane, then add 0.3mL of triethylamine, slowly add 0.2g of acryloyl chloride and 0.2g (2.2mmol) of dichloromethane in an ice bath 5mL, continue to react at room temperature for 6 hours after dropping. After the reaction was ...
Embodiment 2
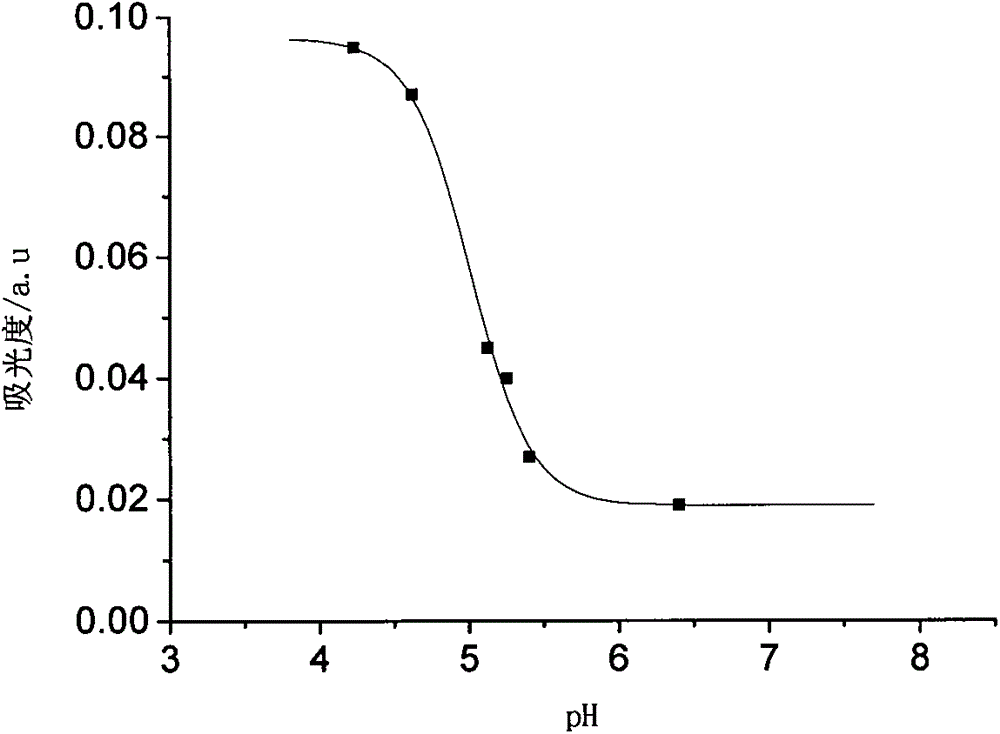
[0034] (1) Draw the ultraviolet-visible absorption spectrum of the polymeric pH probe containing the rhodamine lactam group obtained in Example 1 at different pHs, the concentration is 0.16mg / mL, the abscissa is pH, and the ordinate is absorbance ,have to figure 1 ;
[0035] It can be seen from the figure that when the probe is at pH=4.2-6.5, its absorbance value will change suddenly, and it can be seen that the color response range of the probe solution is at pH=4.2-6.5.
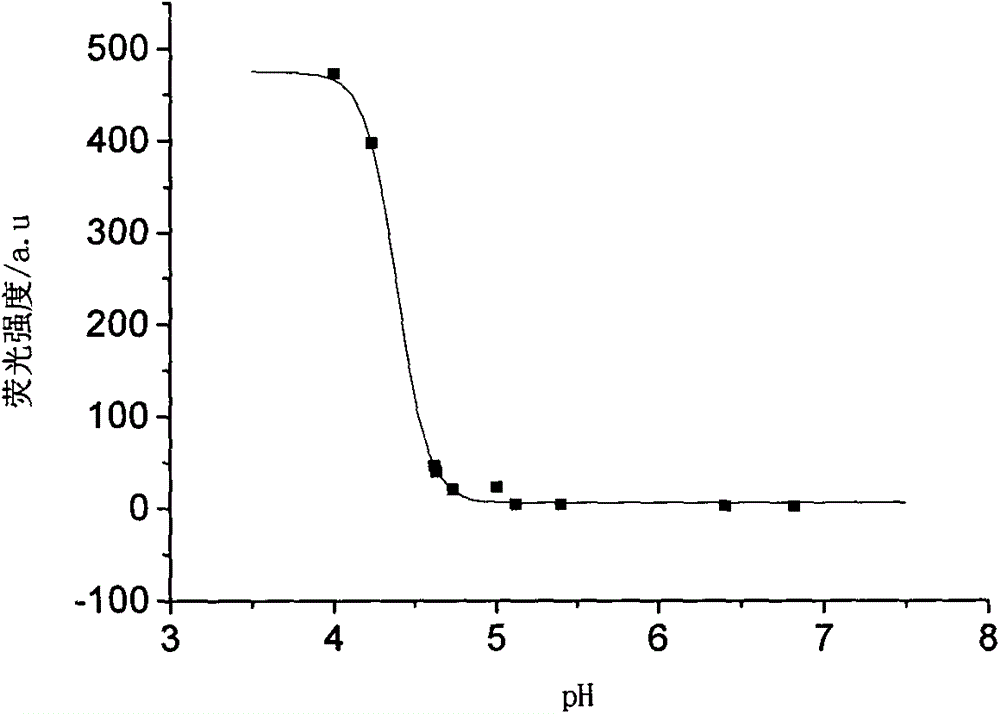
[0036] (2) Draw the fluorescence emission spectrum of the polymer pH probe containing the rhodamine lactam group gained in Example 1 at different pHs in aqueous solution, the concentration is 0.16mg / mL, the abscissa is pH, and the ordinate is fluorescence intensity, have to figure 2 .
[0037] It can be seen from the figure that when the probe is at pH=4-5, its fluorescence intensity has a sudden change, and it can be seen that the fluorescence response range is at pH=4-5. And the response interval is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com