Oral composition of supplement
A composition and drug technology, which is applied in the direction of drug combination, anhydride/acid/halide active ingredients, medical preparations containing active ingredients, etc., can solve problems such as differences, increasing the complexity of patients, and the lack of uniformity in dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016]
[0017] Preparation process:
[0018] (1) Pulverize the above raw materials and pass through a 80-100 mesh sieve for later use.
[0019] (2) Mix the prescribed amount of zinc gluconate and the same amount of glucose evenly, and mix them 5 times in increments, and then mix evenly with the prescribed amount of sodium citrate to form a mixed powder 1.
[0020] (3) Mix the mixed powder 1 and the same amount of glucose evenly, then add the mixed powder 1 to the remaining glucose and mix evenly to form the mixed powder 2.
[0021] (4) Mix the sodium chloride and potassium chloride of the prescribed amount evenly, and it is mixed powder 3.
[0022] (5) Take about half the amount of mixed powder 3 and add mixed powder 3, and mix evenly to form mixed powder 4.
[0023] (6) Take the same amount of mixed powder 2 as mixed powder 4, add mixed powder 4, mix evenly, and become mixed powder 5.
[0024] (7) Add the remaining mixed powder 2 to mixed powder 5, and mix evenly to fo...
Embodiment 2
[0032]
[0033] Preparation process:
[0034] (1) Add hot water for injection of 80% of the prescription quantity into the thick preparation tank, add the raw materials of the prescription quantity and stir until they are all dissolved.
[0035] (2) Supplement water for injection to the prescribed amount, take a sample to measure the pH value, it should be controlled at about 4.0, and the content of each component is about 100%.
[0036] (3) After the medicinal solution is sterilized, it is filtered through a filter membrane with a diameter of 0.22 μm.
[0037] (4) Fill the filtrate with 200ml / bottle.
[0038] (5) seal. Save the finished product after passing the inspection.
[0039] Zinc gluconate in the above-mentioned prescription can be used to provide zinc sulfate, zinc citrate, zinc glycyrrhizinate, zinc acetate of equivalent zinc (Zn).
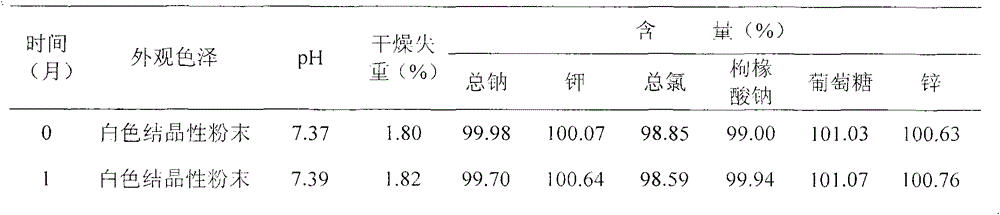
[0040] The stability test data of the sample prepared by above-mentioned embodiment 2 is as follows table 2, as seen from the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com