Co-loaded adriamycin and siRNA (small interfering ribose nucleic acid) carrier capable of removing pegylation and synthesis method thereof
A technology of PEGylation and synthesis method, which is applied in the field of co-loading doxorubicin and siRNA carrier and its synthesis, which can solve the problems of inability to play a therapeutic role, improve the drug effect, increase the effective concentration of the drug, and avoid enzymatic hydrolysis Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
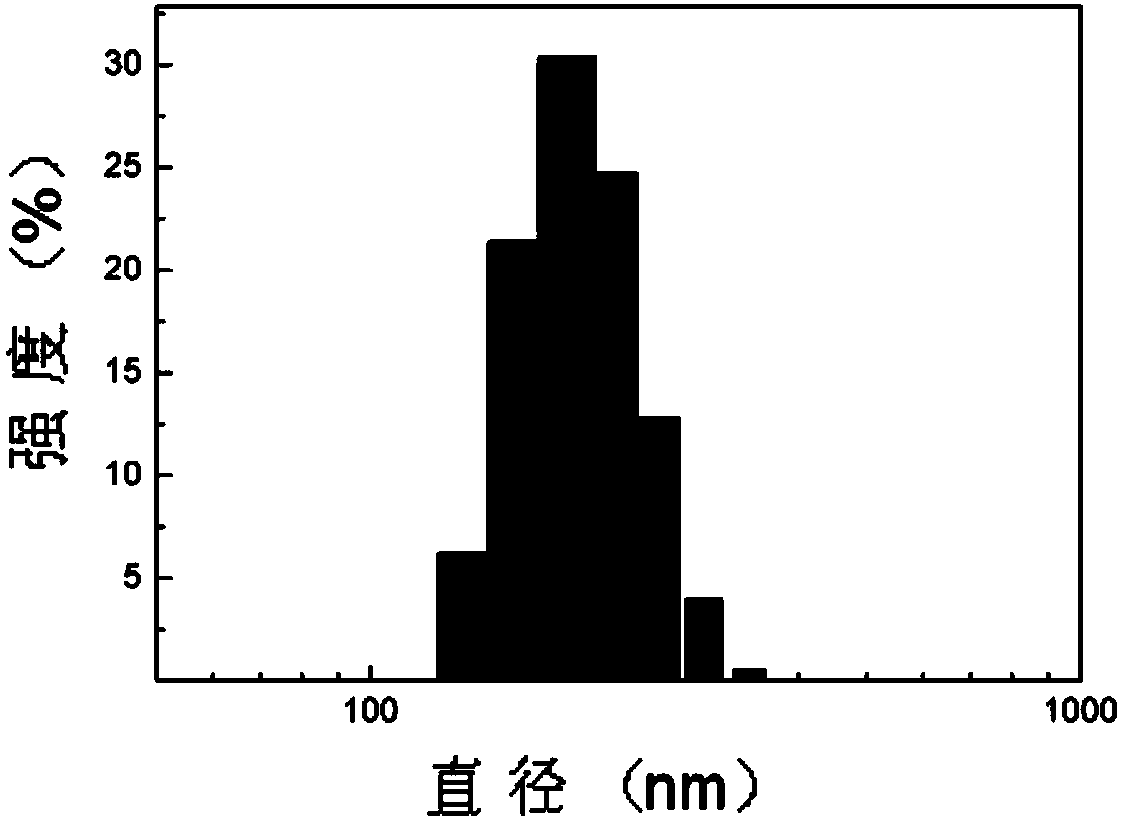
Examples
Embodiment 1
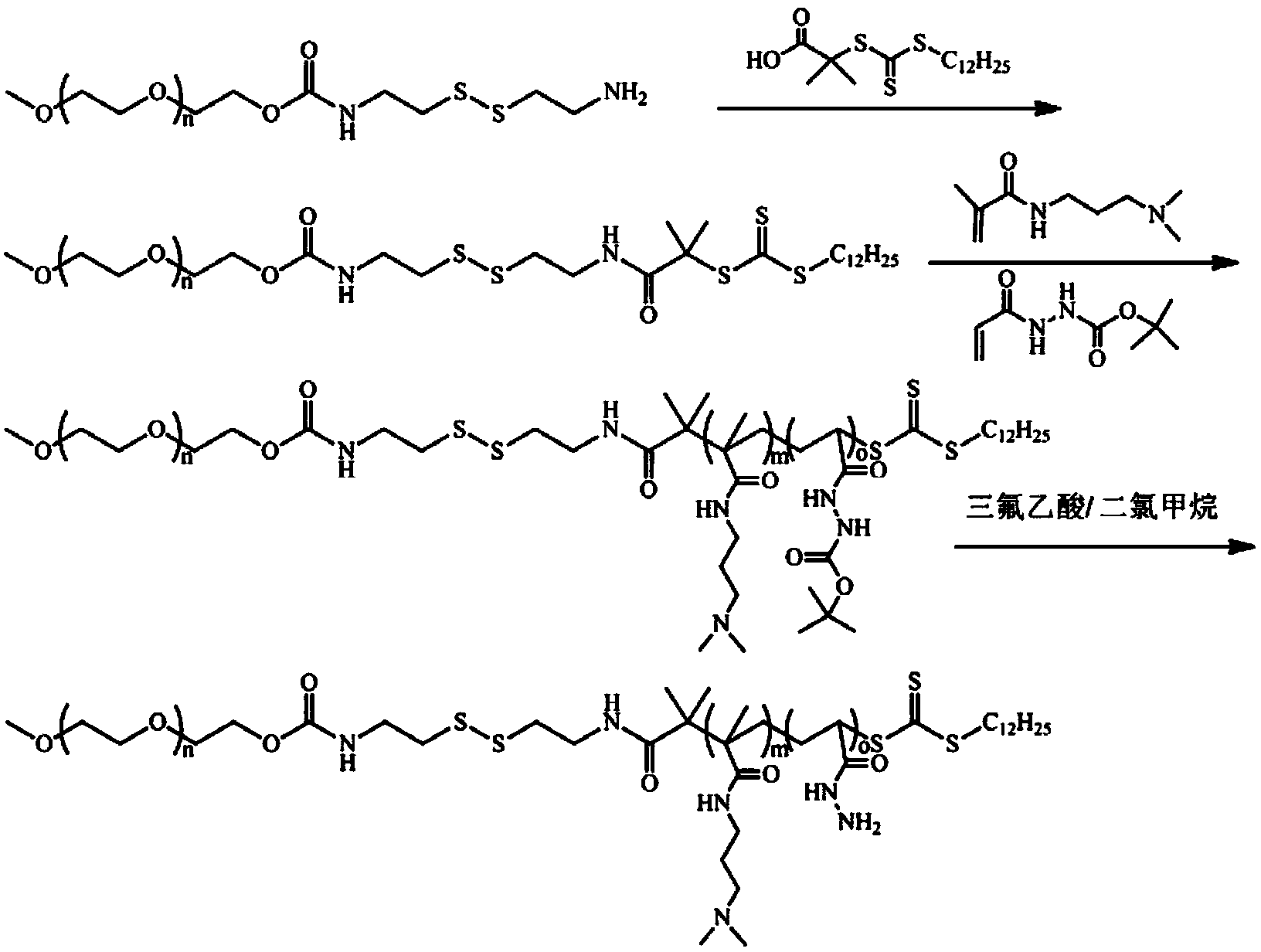
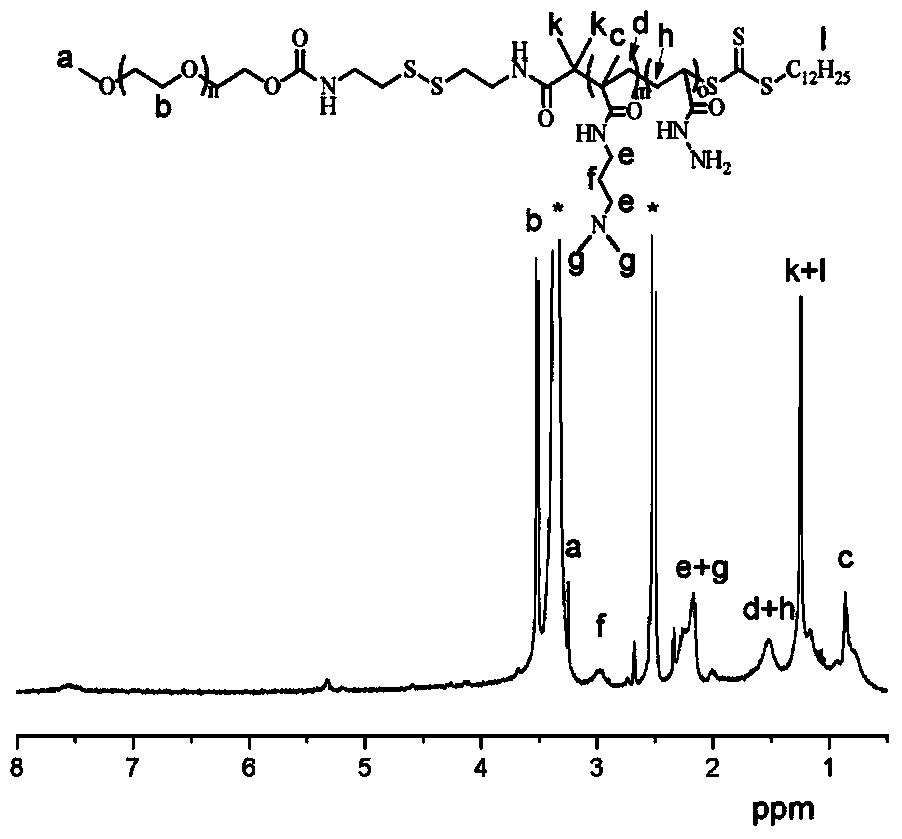
[0042] 1) Synthesis of cystaminated methoxypolyethylene glycol:
[0043] Dissolve dry methoxy-terminated polyethylene glycol (abbreviated as mPEG) with a molecular weight of 800Da, dibutyltin dilaurate and 2,2'-dithiodiethylisocyanate in anhydrous toluene, under nitrogen atmosphere React at 85°C for 48 hours, then precipitate in anhydrous n-hexane for 3 times, and dry the obtained solid in vacuum to obtain 2,2'-dithiodiethylisocyanate-modified mPEG; wherein, the added mPEG 1. The molar ratio of dibutyltin dilaurate to 2,2'-dithiodiethylisocyanate is 1:0.02:4, and 10g of mPEG is dissolved in every 100ml of anhydrous toluene;
[0044] Dissolve 2,2'-dithiodiethylisocyanate-modified mPEG in distilled water, then react at 60°C for 6 hours, and finally dialyze and freeze-dry the obtained reaction solution to obtain white powder cystamination Methoxy-terminated polyethylene glycol (abbreviated as cystaminated mPEG); wherein, 5 g of 2,2'-dithiodiethylisocyanate-modified mPEG was diss...
Embodiment 2
[0060] 1) Synthesis of cystaminated methoxypolyethylene glycol:
[0061] Dissolve dry methoxy-terminated polyethylene glycol (abbreviated as mPEG) with a molecular weight of 2000Da, dibutyltin dilaurate and 2,2'-dithiodiethylisocyanate in anhydrous toluene, under nitrogen atmosphere React at 85°C under a nitrogen atmosphere for 48 hours, then precipitate in anhydrous n-hexane for 3 times, and dry the obtained solid in vacuum to obtain 2,2'-dithiodiethylisocyanate-modified mPEG; wherein, the added The molar ratio of mPEG, dibutyltin dilaurate and 2,2'-dithiodiethylisocyanate is 1:0.04:4; 20g of mPEG is dissolved in 100ml of anhydrous toluene;
[0062] Dissolve 2,2'-dithiodiethylisocyanate-modified mPEG in distilled water, then react at 60°C for 6 hours, and finally dialyze and freeze-dry the obtained reaction solution to obtain white powder cystamination Methoxy-terminated polyethylene glycol (abbreviated as cystaminated mPEG); wherein, 15 g of 2,2'-dithiodiethylisocyanate-mod...
Embodiment 3
[0074] 1) Synthesis of cystaminated methoxypolyethylene glycol:
[0075] Dissolve dry methoxy-terminated polyethylene glycol (abbreviated as mPEG) with a molecular weight of 2000Da, dibutyltin dilaurate and 2,2'-dithiodiethylisocyanate in anhydrous toluene, under nitrogen atmosphere React at 85°C under a nitrogen atmosphere for 48 hours, then precipitate in anhydrous n-hexane for 3 times, and dry the obtained solid in vacuum to obtain 2,2'-dithiodiethylisocyanate-modified mPEG; wherein, the added The molar ratio of mPEG, dibutyltin dilaurate and 2,2'-dithiodiethylisocyanate is 1:0.05:5; 10g of mPEG is dissolved in 100ml of anhydrous toluene;
[0076] Dissolve 2,2'-dithiodiethylisocyanate-modified mPEG in distilled water, then react at 60°C for 6 hours, and finally dialyze and freeze-dry the obtained reaction solution to obtain white powder cystamination Methoxy-terminated polyethylene glycol (abbreviated as cystaminated mPEG); wherein, 10 g of 2,2'-dithiodiethylisocyanate-mod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com