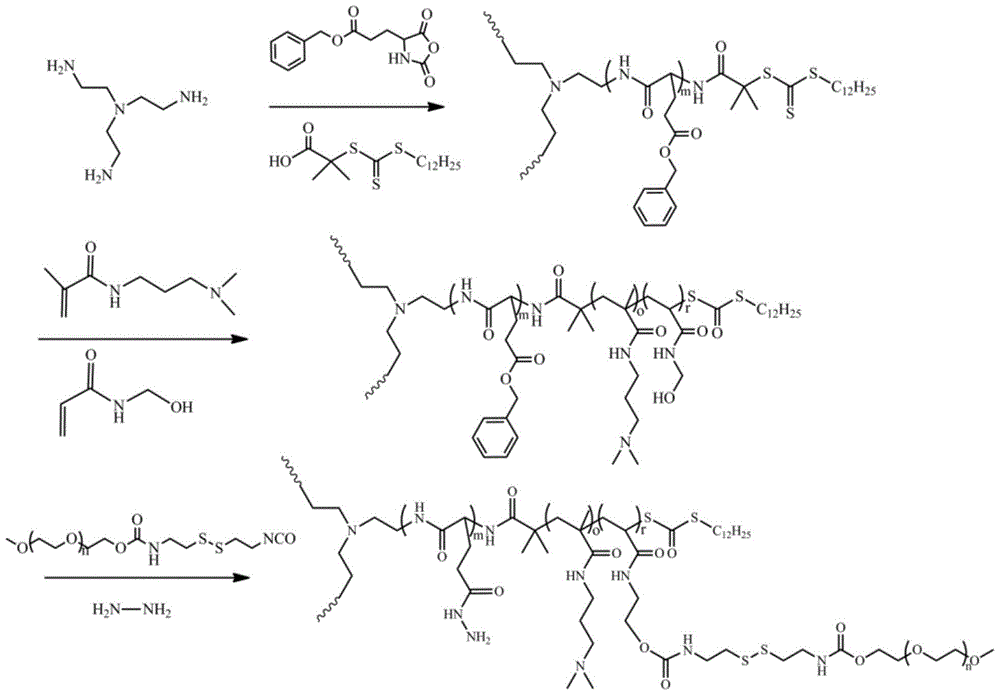
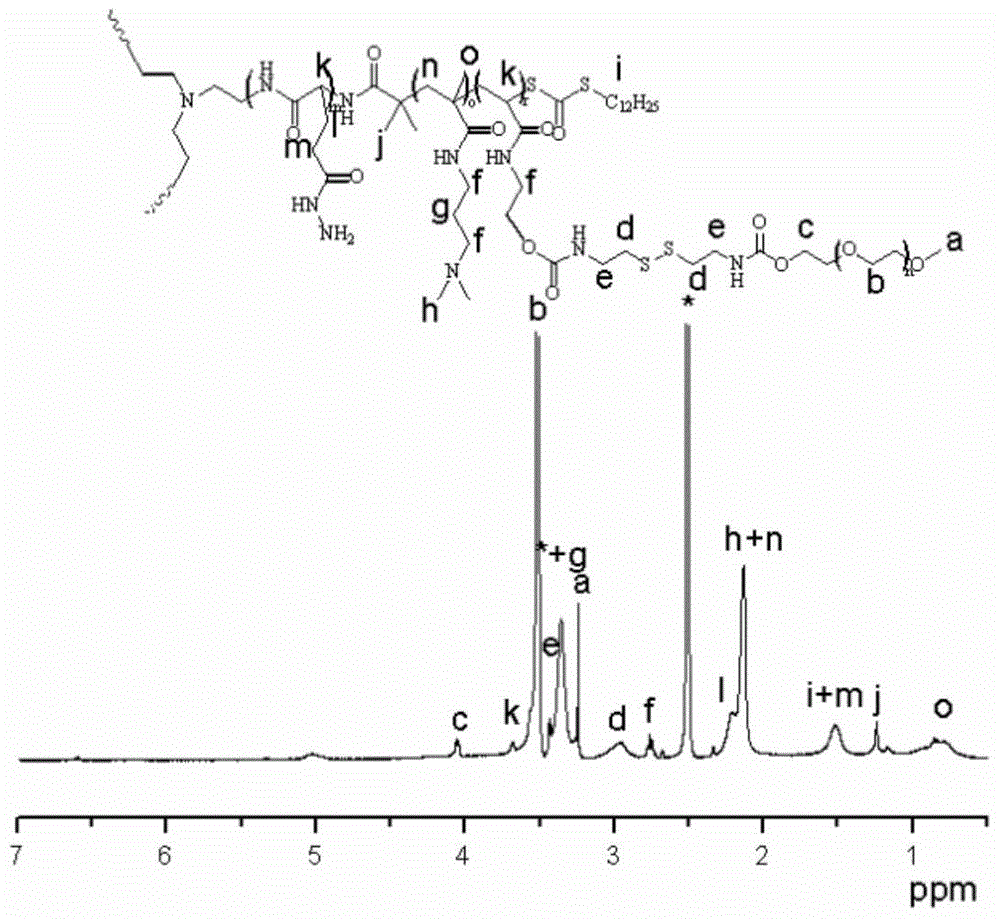
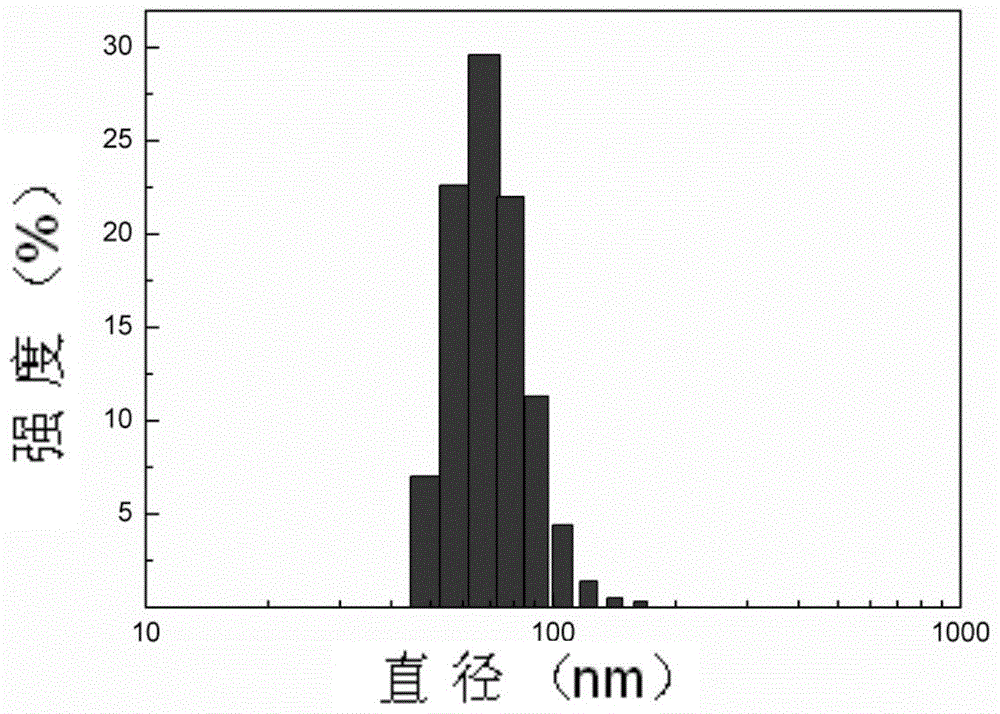
A kind of three-arm star-shaped hydrophilic copolymer and its synthesis method and application
A technology of a hydrophilic copolymer and a synthesis method, applied in the field of biomedical materials, can solve the problems of slow drug release rate and difficulty in fully exerting drug efficacy, and achieve the effects of improving efficacy, low critical micelle concentration, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1) Synthesis of γ-benzyl ester-L-glutamic acid-N-carboxyl anhydride (BLG-NCA):
[0048] Add L-glutamic acid-γ-benzyl ester into anhydrous tetrahydrofuran, then add triphosgene under nitrogen atmosphere, then react at 50°C until a clear solution is formed, then concentrate under reduced pressure to remove most of the solvent to concentrate the solvent, and finally to Add anhydrous n-hexane to the reaction system obtained after concentration to precipitate, and recrystallize the obtained precipitate with anhydrous n-hexane to obtain γ-benzyl ester-L-glutamic acid-N-carboxyl anhydride (BLG-NCA) ; Wherein, the molar ratio of L-glutamic acid-γ-benzyl ester to triphosgene is 1:0.35, and 10g of L-glutamic acid-γ-benzyl ester is dissolved in every 100 milliliters of anhydrous tetrahydrofuran;
[0049] 2) Synthesis of three-arm poly(L-glutamate-γ-benzyl ester) (tPBLG):
[0050] Reflux tris(2-aminoethyl)amine through calcium hydride for 12 hours, and distill under reduced pressu...
Embodiment 2
[0067] 1) Same as step 1) in Example 1.
[0068] 2) Synthesis of three-arm poly(L-glutamate-γ-benzyl ester) (tPBLG):
[0069] Reflux tris(2-aminoethyl)amine through calcium hydride for 12 hours, and distill under reduced pressure under nitrogen atmosphere to obtain anhydrous tris(2-aminoethyl)amine, then dissolve anhydrous tris(2-aminoethyl)amine In anhydrous chloroform, add BLG-NCA under a nitrogen atmosphere, stir to form a uniform solution, and react at 40°C for 60 hours; after the reaction, the obtained reaction solution is precipitated with cold ether and washed with ethanol, and vacuum-dried to obtain a white powder tPBLG; wherein, the molar ratio of anhydrous tris(2-aminoethyl)amine and BLG-NCA is 1:60, and 5g of BLG-NCA is dissolved in every 100 milliliters of anhydrous chloroform;
[0070] 3) Synthesis of three-arm poly(L-glutamic acid-γ-benzyl ester) macromolecular RAFT chain transfer agent:
[0071] The tPBLG was azeotropically dewatered with toluene under nitroge...
Embodiment 3
[0080] 1) Same as step 1) in Example 1.
[0081] 2) Synthesis of three-arm poly(L-glutamate-γ-benzyl ester) (tPBLG):
[0082] Reflux tris(2-aminoethyl)amine through calcium hydride for 12 hours, and distill under reduced pressure under nitrogen atmosphere to obtain anhydrous tris(2-aminoethyl)amine, then dissolve anhydrous tris(2-aminoethyl)amine In anhydrous tetrahydrofuran, add BLG-NCA under a nitrogen atmosphere, stir to form a uniform solution, and react at 40°C for 48 hours; after the reaction, the obtained reaction solution is precipitated with cold ether and washed with ethanol, and vacuum-dried to obtain a white powder tPBLG; wherein, the molar ratio of anhydrous tris(2-aminoethyl)amine and BLG-NCA is 1:45, and 5g of BLG-NCA is dissolved in every 100 milliliters of anhydrous tetrahydrofuran;
[0083] 3) Synthesis of three-arm poly(L-glutamic acid-γ-benzyl ester) macromolecular RAFT chain transfer agent:
[0084]The tPBLG was azeotropically dewatered with toluene unde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com