A core-shell structured chitosan-based nano-prodrug that co-loads doxorubicin and platinum drugs and its preparation method and application
A technology of platinum-based drugs and core-shell structure, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations of non-active ingredients. Clear, simple effect of drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
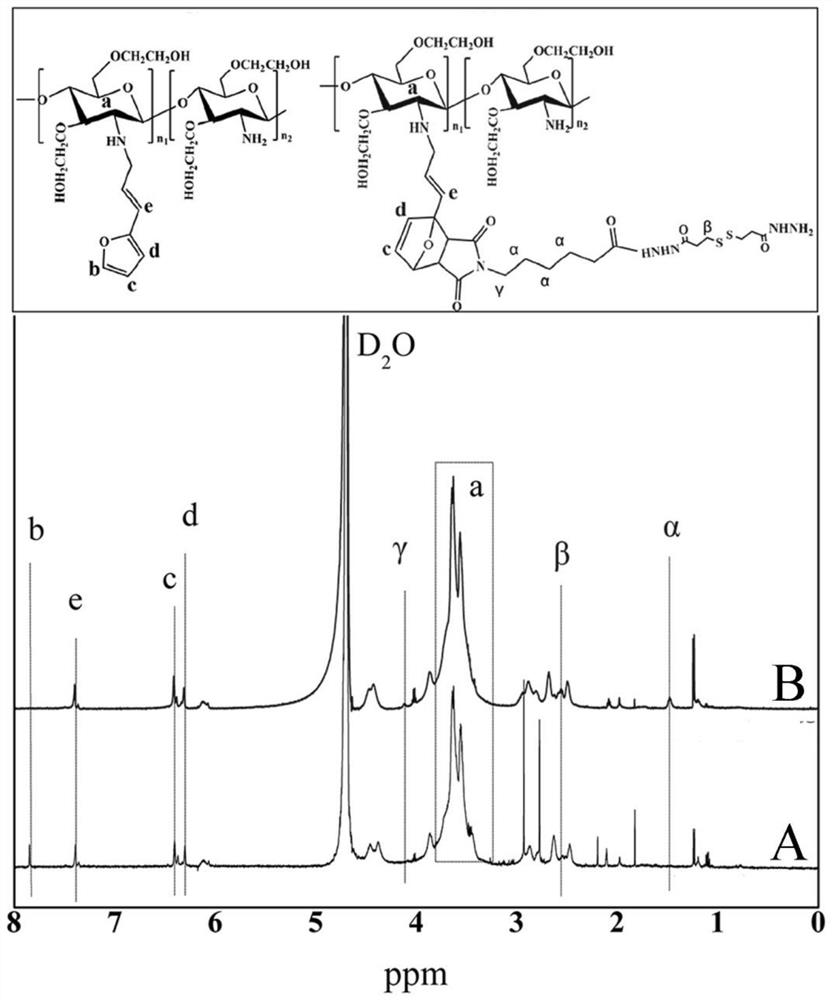
[0043]A preparation method of a core-shell structured chitosan-based nano-prodrug co-loaded with doxorubicin and platinum drugs of the present invention is as follows: firstly, 3,3'-dithiodipropionyl Hydrazine is bonded to chitosan derivatives to obtain hydrazide chitosan derivatives. At the same time, doxorubicin is linked to 3,3'-dithiodipropionyl hydrazide through a hydrazone bond to obtain hydrazide doxorubicin Then the aqueous solution of hydrazidated chitosan derivatives, hydrazidated doxorubicin and platinum drugs is mixed evenly, and the core-shell structure type chitosan-based nano-prodrug is obtained through the self-assembly process, which specifically includes the following steps:
[0044] (1) Preparation of hydrazidated chitosan derivatives: Aldehydated furan solution is added dropwise to water-soluble chitosan solution, aldehyde groups and amino groups undergo a Schiff base reaction, and are reduced by sodium cyanoborohydride to form Furanated chitosan derivative...
Embodiment 1
[0066] (1) Preparation of hydrazidated hydroxyethyl chitosan: 3-(2-furyl) acrolein solution with a mass concentration of 0.33% was added dropwise to 1.0% hydroxyethyl chitosan (MW = 170kDa) solution and mix well, the molar ratio of 3-(2-furyl) acrolein to amino group in hydroxyethyl chitosan is 10:8, at 30℃ and N 2 React under the atmosphere for 12h, then add the sodium cyanoborohydride solution with a mass concentration of 0.6%. The amount of sodium cyanoborohydride is 1.3 times the number of Schiff base bonds. 2 The reduction reaction was continued for 12 hours under the atmosphere to form a furanated hydroxyethyl chitosan crude product, which was dialyzed in deionized water for 72 hours with a dialysis bag with a molecular weight cut-off of 3500 Da and then freeze-dried to obtain furanated hydroxyethyl chitosan;
[0067] Be that the mass concentration of 1.0% maleimide caproic acid solution is 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide solution and 0.57mmol mass concent...
Embodiment 2
[0079] (1) Preparation of hydrazidated hydroxyethyl chitosan: 3-(2-furyl) acrolein solution with a mass concentration of 0.33% was added dropwise to 1.0% hydroxyethyl chitosan (MW = 200kDa) solution and mix well, the molar ratio of 3-(2-furyl) acrolein to amino group in hydroxyethyl chitosan is 10:1, at 30 ℃ and N 2 React under the atmosphere for 12h, then add a sodium cyanoborohydride solution with a mass concentration of 0.6%, the amount of sodium cyanoborohydride is 1.1 times the number of Schiff base bonds, at 20 ° C and N 2 The reduction reaction was continued for 1 h under the atmosphere to form a furanated hydroxyethyl chitosan crude product, which was dialyzed in deionized water for 72 h with a dialysis bag with a molecular weight cut-off of 3500 Da and then freeze-dried to obtain furanated hydroxyethyl chitosan;
[0080] Be that the mass concentration of 1.0% maleimide caproic acid solution is 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide solution and 0.57mmol mass c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
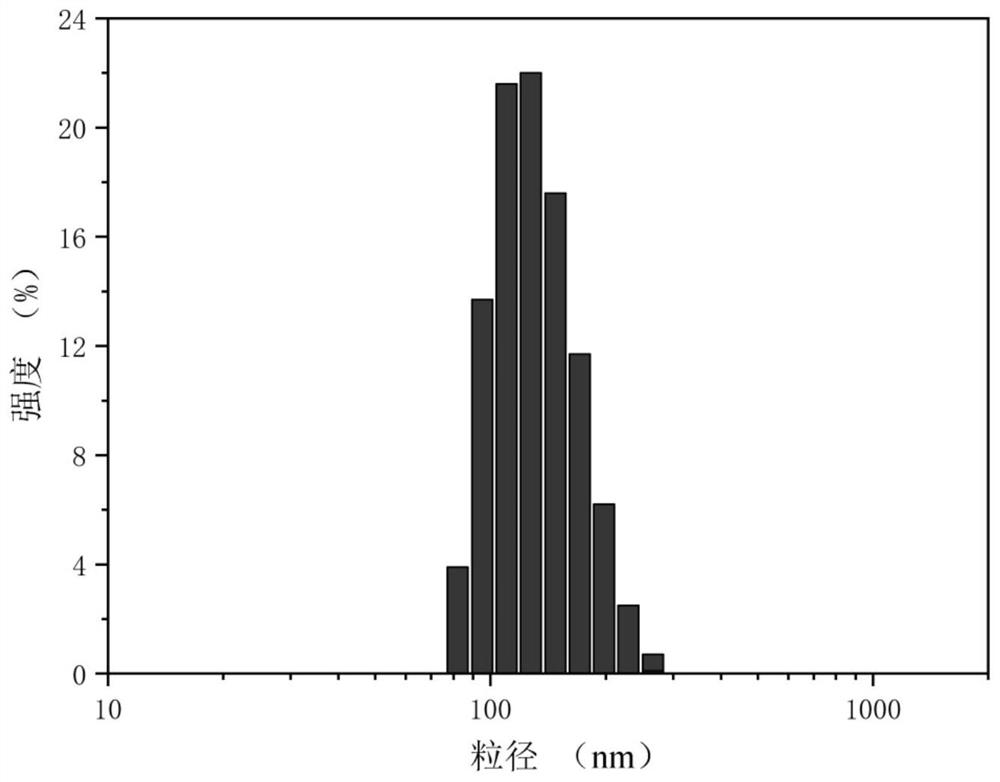
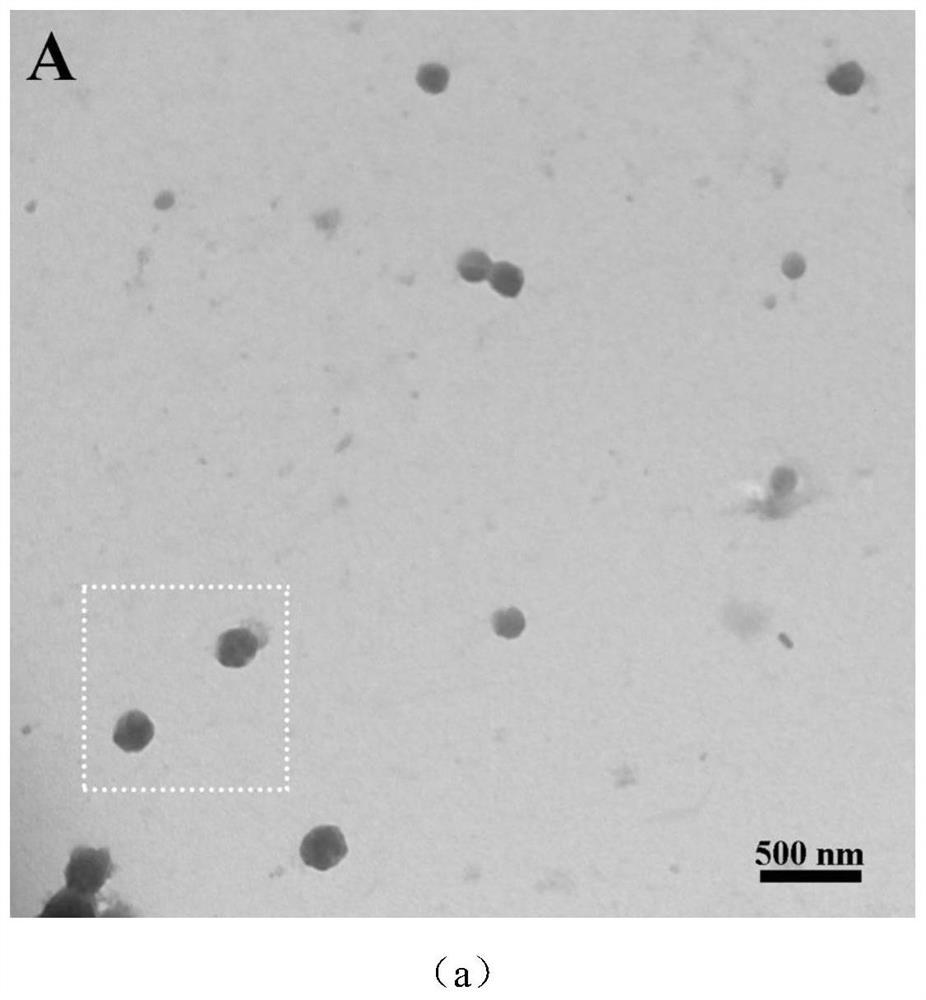
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com