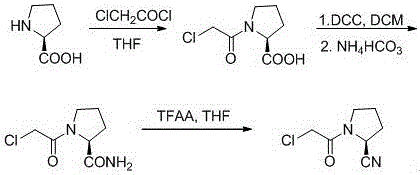
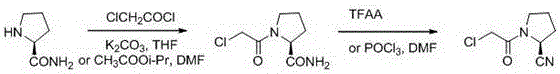
A kind of synthetic method of (s)-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile
A synthetic method, the technology of chloroacetyl group, applied in the direction of organic chemistry, etc., can solve the problems of easy moisture absorption, slow reaction speed, inconvenient preparation, storage and feeding, etc., and achieve low water absorption, prolonged reaction time and convenient preparation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
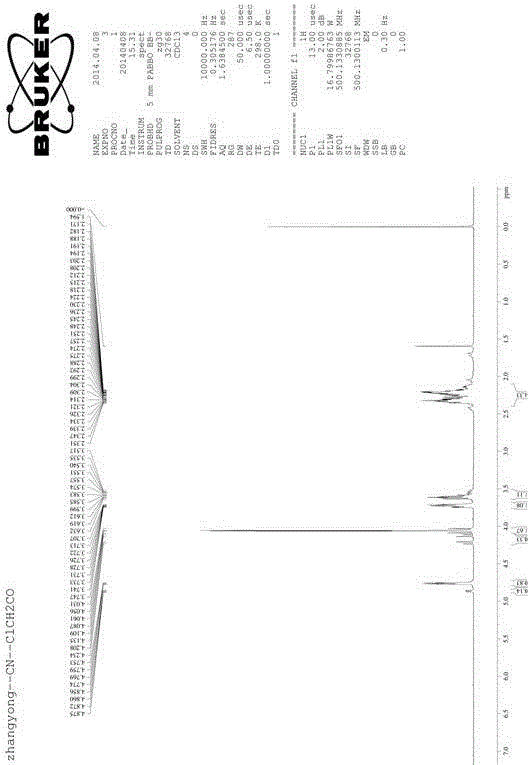
[0048] Take a reaction bottle, add ( S )-2-cyanopyrrolidine p-toluenesulfonate (40g, 0.15mol) and dichloromethane (200mL), cooled to -10°C, added triethylamine (0.15mol) dropwise, and stirred to obtain a clear solution; Chloroacetyl chloride (0.225mol) was added dropwise to the clarified solution, and the temperature was controlled at -5°C. After the dropwise addition was completed, the stirring was continued until the reaction was complete, and 100mL of water was added to quench the reaction, due to ( S )-2-cyanopyrrolidine p-toluenesulfonate reacts with triethylamine to generate pyrrolidine-2-carbonitrile, and the pyrrolidine-2-carbonitrile is detected in real time by thin layer chromatography. When pyrrolidine-2 -The disappearance of the color development of formonitrile means that the reaction is complete; after standing still and quenching, the solution is separated, the water phase is removed, and the organic phase is saturated with NaHCO 3 The solution was washed 2 tim...
Embodiment 2
[0050] Take a reaction bottle, add ( S )-2-cyanopyrrolidine p-toluenesulfonate (40g, 0.15mol), dichloromethane (400mL), cooled to -5°C, added dropwise triethylamine (0.3mol), and stirred to obtain a clear solution. Chloroacetyl chloride (0.3mol) is then added dropwise to the clear solution, the temperature is controlled below 0°C, after the dropwise addition is complete, continue to stir until the reaction is complete, and add 100mL of water to quench the reaction; Aqueous phase, organic phase with saturated NaHCO 3 The solution was washed 2 times, each time saturated with NaHCO 3 The volume of the solution was 100mL; after washing, let stand to separate the liquid, remove the water phase, dry the washed organic phase with anhydrous magnesium sulfate, and concentrate to obtain 22.5 g of white solid, with a yield of 86.7%. Detected by nuclear magnetic resonance, the resulting white solid is ( S )-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile, the purity determined by HPLC met...
Embodiment 3
[0052] Take a reaction bottle, add ( S )-2-cyanopyrrolidine p-toluenesulfonate (40g, 0.15mol), dichloromethane (400mL), cooled to 10°C, added dropwise triethylamine (0.45mol), and stirred to obtain a clear solution. Chloroacetyl chloride (0.45mol) is then added dropwise to the clear solution, the temperature is controlled below 10°C, after the dropwise addition is complete, continue to stir until the reaction is complete, and add 100mL of water to quench the reaction; Aqueous phase, organic phase with saturated NaHCO 3 The solution was washed 2 times, each time saturated with NaHCO 3 The volume of the solution was 100 mL; after washing, it was left standing to separate the liquid, the water phase was removed, the washed organic phase was dried with anhydrous magnesium sulfate, and concentrated to obtain 23 g of white solid, with a yield of 88.6%. Detected by nuclear magnetic resonance, the resulting white solid is ( S )-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile, the puri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com