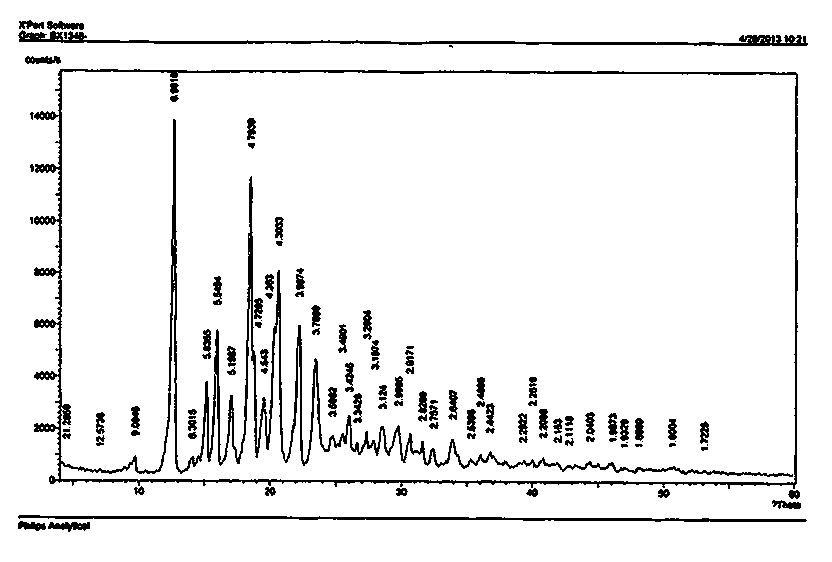
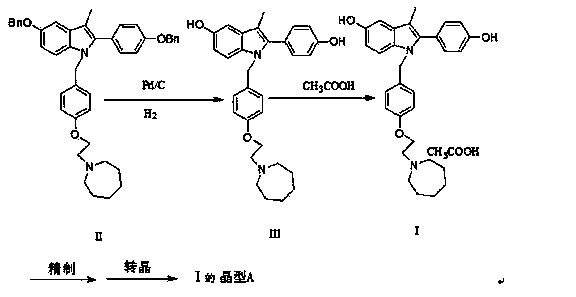
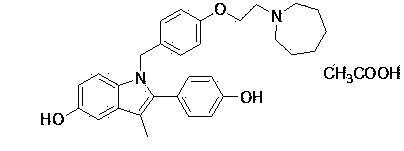
Preparation method of bazedoxifene acetate crystal form A
A technology of bazedoxifene acetate and crystal form, applied in the field of chemical pharmacy, can solve problems such as difficulty, and achieve the effects of good reproducibility, obvious advantages in preparation process, and high purity and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1: Preparation of bazedoxifene free base III
[0061]Add distilled water to 10% Pd / C and wash twice, filter off part of distilled water to control its water content at 50~56%; 20g (0.031mol) 5-benzyloxy-2-(4-benzyloxyphenyl )-3-methyl-1-[4-(2-azepan-1-ylethoxy) benzyl]-1H-indole II was dissolved in 120ml ethyl acetate and 40ml ethanol, added 13.5 g The above Pd / C is heated to 50~55°C, stirred and reacted at 0.1MPa for 4~5h, and the reaction is completed by TLC monitoring. Filter out Pd / C under the protection of argon, add trace vitamin C and acetic acid to the solution directly at 50~55°C to form acetate, the yield is 100%
Embodiment 2
[0062] Embodiment 2: Preparation of bazedoxifene acetate I
[0063] Put the filtrate in Example 1 in a 250ml reaction bottle, add a small amount of vitamin C, vacuumize, replace with argon 1~2 times, heat up to 50°C~55°C, add 2.06g (0.034mol) of glacial acetic acid dropwise to the reaction solution, stirred at 50°C~55°C for 1~1.5h, stirred at room temperature for 2h, filtered to obtain an off-white solid, washed the solid with ethanol for 1~2 times, and dried to obtain 12.11g of off-white solid, yield 83.7%, HPLC: 99.67 %, mp177-180°C.
Embodiment 3
[0064] Embodiment 3: Refining of bazedoxifene acetate I
[0065] Add 83.8ml of ethanol, 51.2ml of acetone, 10.48ml of water and a small amount of vitamin C to the dried 12.11g of solid, heat to reflux until all the solid is dissolved, then slowly cool down to room temperature, and put it in the refrigerator (-5~-0°C) After overnight, it was filtered and dried to obtain 9.81 g of off-white solid, yield 81.2%, HPLC: 99.86%, mp 178-180°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com