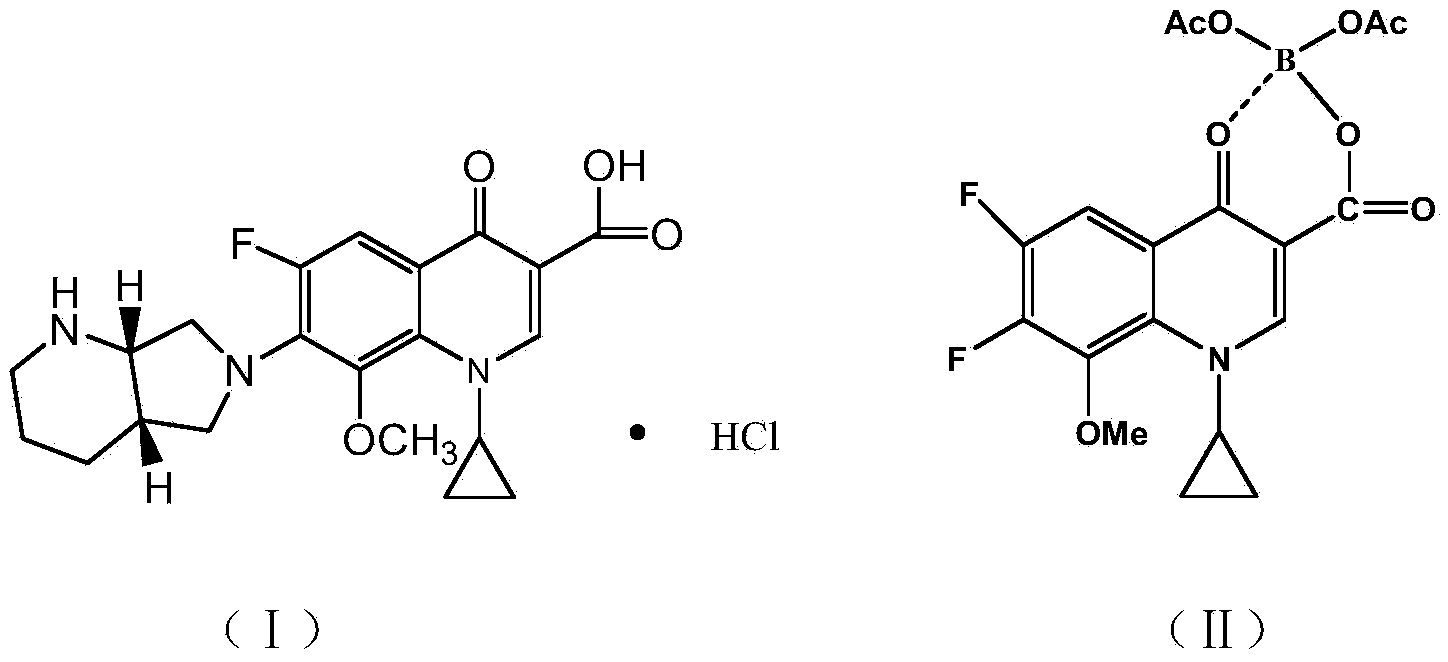
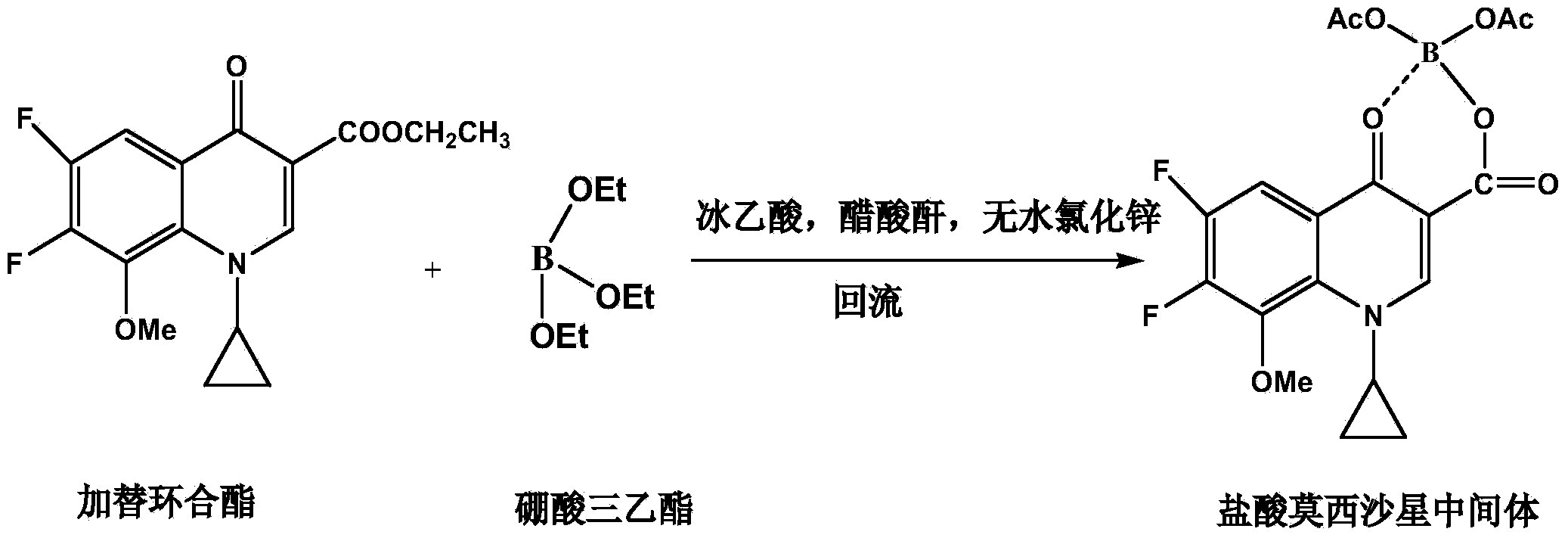
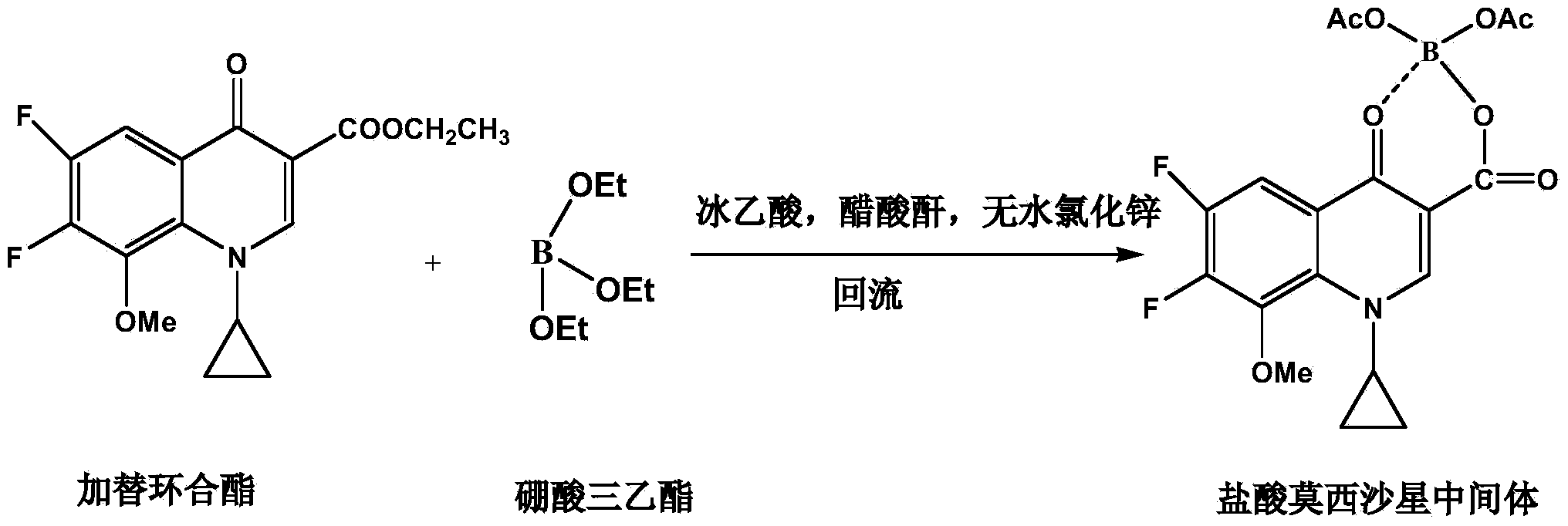
Synthesis method of moxifloxacin hydrochloride intermediate
A technology of moxifloxacin hydrochloride and a synthesis method is applied in chemical instruments and methods, compounds containing elements of Group 3/13 of the periodic table, organic chemistry, etc., and can solve the problems of many steps, complex synthesis process, complicated operation and the like, To achieve the effect of easy operation and simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: a kind of synthetic method of moxifloxacin hydrochloride intermediate, it comprises the following steps:
[0021] S1. Chelation reaction: Add 280g acetic anhydride and 120g glacial acetic acid in turn in the reaction kettle, stir and add 5g anhydrous zinc chloride; heat up, add 80g triethyl borate dropwise when the temperature in the reaction kettle is 40°C, drop Continue to keep stirring at the temperature for 3 hours after the addition is completed, add 150 g gaticycline ester after completion, raise the temperature to 70°C, and track the reaction with TLC until the conversion of gaticyclate is complete;
[0022] S2. Post-processing: After the reaction, the temperature in the kettle was lowered to 15°C, stirred and crystallized for 20 hours, centrifugally filtered, and the obtained crystalline solid was beaten twice with purified water, each time for 20 minutes, and the slurry was filtered, then beaten with ethanol for 20 minutes, and centrifugally filter...
Embodiment 2
[0023] Embodiment 2: a kind of synthetic method of moxifloxacin hydrochloride intermediate, it comprises the following steps:
[0024] S1. Chelation reaction: Add 280g acetic anhydride and 120g glacial acetic acid in turn in the reaction kettle, stir and add 5g anhydrous zinc chloride, heat up, when the temperature in the reaction kettle is 50°C, add 120g triethyl borate dropwise, drop Continue stirring for 5 hours after the addition is complete, add 150 g gaticycline ester after completion, raise the temperature to 75°C, and track the reaction with TLC until the conversion of gaticyclate is complete;
[0025] S2. Post-processing: After the reaction is completed, lower the temperature in the kettle to 25°C and stir for 25 hours to crystallize, then centrifuge and filter the obtained crystalline solid with purified water for 2 times, 30 minutes each time, filter the slurry, and then use ethanol to beat for 30 minutes. After centrifugal filtration, the obtained solid was dried w...
Embodiment 3
[0026] Embodiment 3: a kind of synthetic method of moxifloxacin hydrochloride intermediate, it comprises the following steps:
[0027] S1. Chelation reaction: Add 300g acetic anhydride and 120g glacial acetic acid in turn in the reactor, stir and add 5g anhydrous zinc chloride; heat up, when the temperature in the reactor is 58°C, add 150g triethyl borate dropwise, drop Continue stirring for 3.5 hours after the addition is complete, add 150 g gaticycline ester after completion, raise the temperature to 90°C, track the reaction with TLC until the conversion of gaticyclate is complete;
[0028] S2. Post-processing: After the reaction, the temperature in the kettle was lowered to 18°C, stirred and crystallized for 28 hours, centrifugally filtered, and the obtained crystalline solid was beaten twice with purified water, each time for 23 minutes, and the slurry was filtered, then beaten with ethanol for 25 minutes, and centrifugally filtered again. The obtained solid was dried with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com