Flunarizine hydrochloride composition capsule and preparation method thereof
The technology of flunarizine hydrochloride and composition is applied in the field of flunarizine hydrochloride composition capsule and preparation thereof, which can solve the problems of poor drug fluidity, unsatisfactory drug dissolution rate, easy recoagulation and the like, and achieves the dissolution rate and The effect of fluidity improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: preparation flunarizine hydrochloride composition capsule of the present invention
[0027] Ultrafinely pulverize 11.8 parts of flunarizine hydrochloride to a diameter of less than 10 μm, then add it to polyethylene glycol 6000 in a molten state, fully disperse it with a homogenizer, quickly put it into the freeze-drying box of a freeze dryer, freeze Dried to a waxy solid, crushed through a 150-mesh sieve for subsequent use;
[0028] Starch, lactose, talc and magnesium stearate are dried and crushed through a 150-mesh sieve for later use;
[0029] Mix 75 parts of lactose and 47.2 parts of starch evenly with the pulverized waxy solid by an equal amount addition method, then add 25 parts of talcum powder and 1 part of magnesium stearate and mix evenly, then fill them into hollow capsules to obtain Flunarizine hydrochloride composition capsules.
Embodiment 2
[0030] Embodiment 2: Dissolution testing
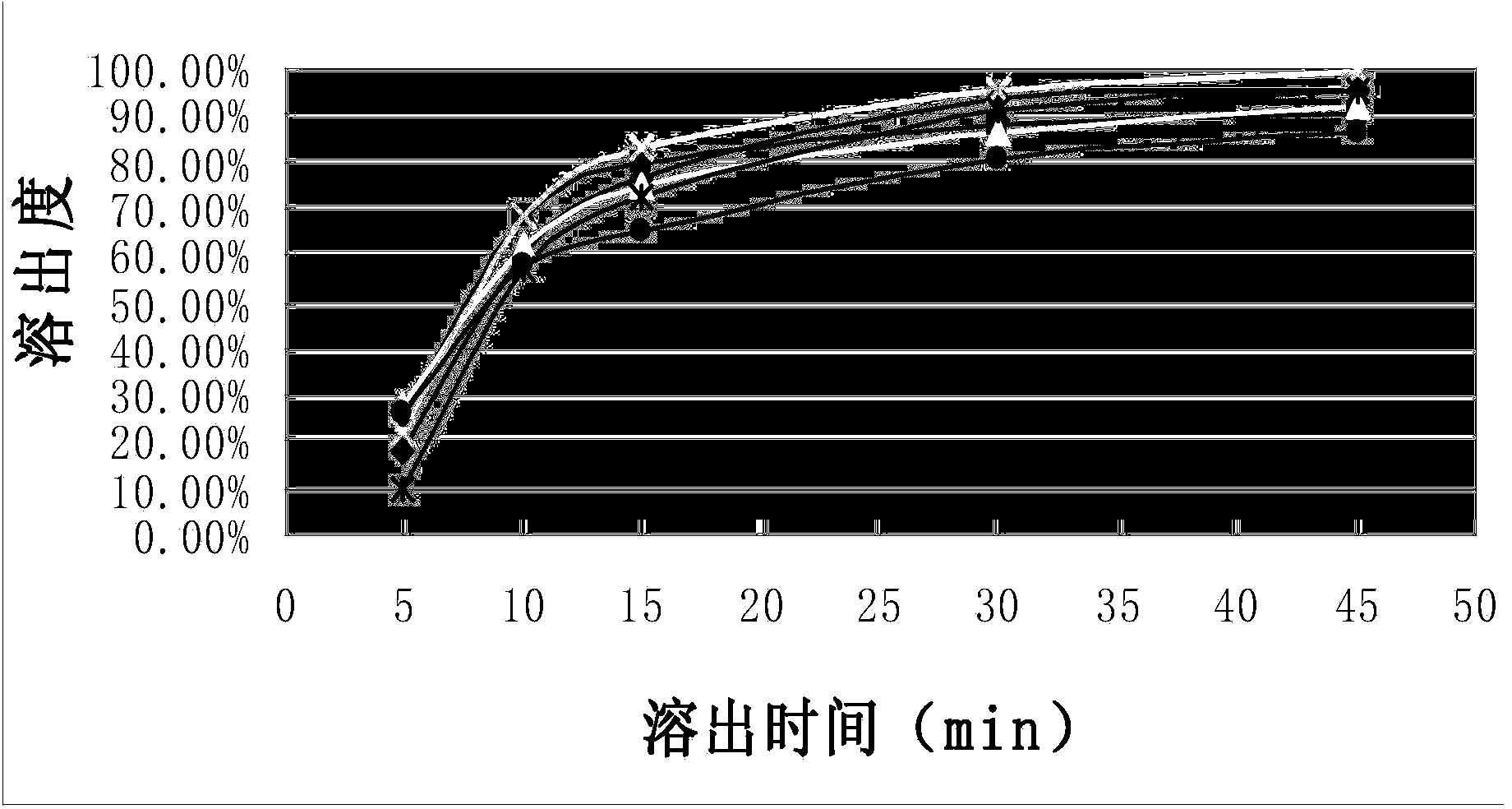
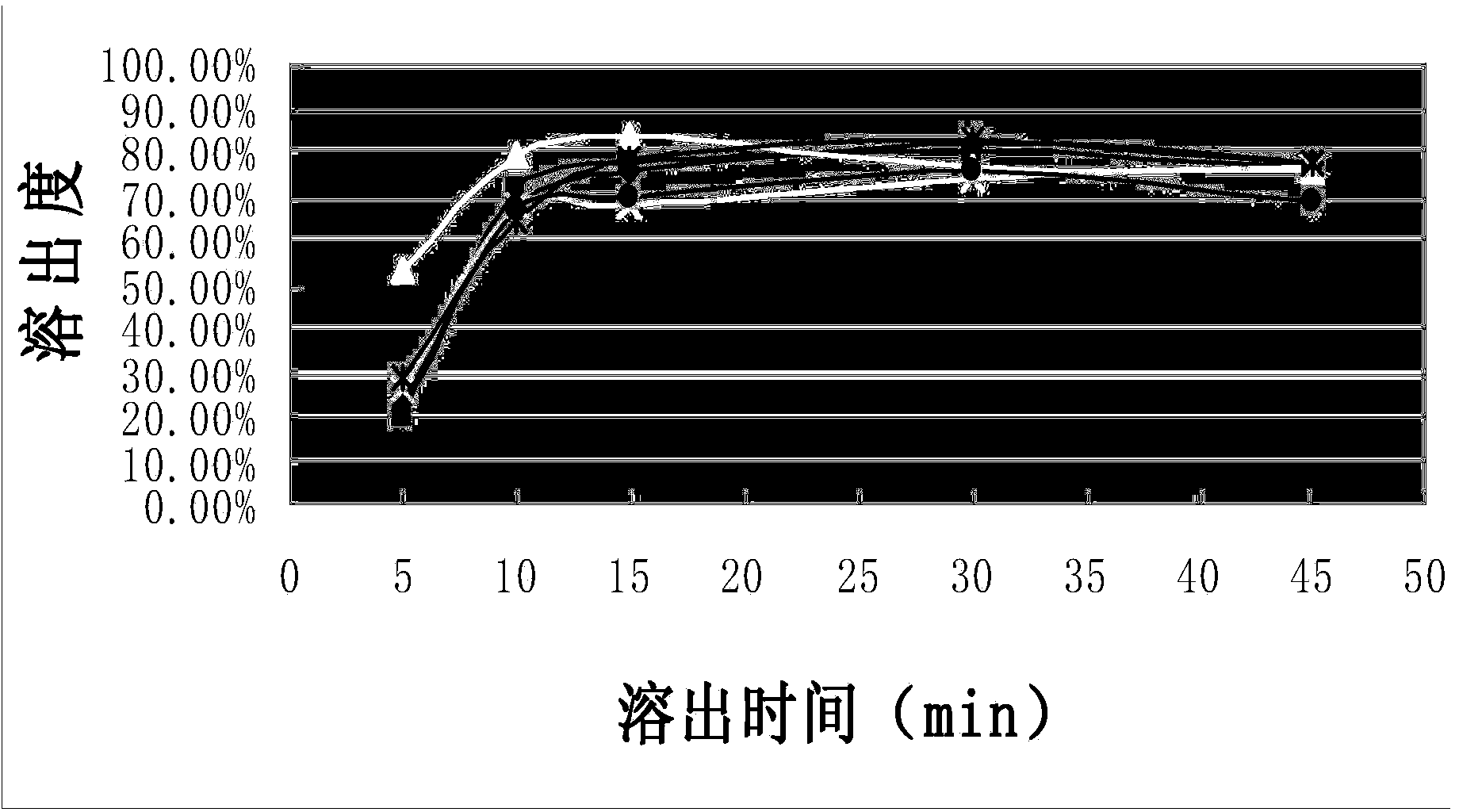
[0031] The dissolution curve of the capsules of the composition of Example 1 was measured according to the standard of Chinese Pharmacopoeia 2010 edition, which was repeated 6 times. Simultaneously according to CN1839838A embodiment 1-embodiment 3 (each repeats 2 times) method to prepare capsule and measure dissolution curve, the results are shown in figure 1 and figure 2 , see Table 1 and Table 2 for the corresponding tabular data.
[0032] Compared figure 1 and figure 2 It can be seen that the composition capsule prepared by the present invention gradually releases the medicinal ingredients as time prolongs, and the stripping effect is stable and better; while the contrast product presents a tendency to increase and then decline as time goes on, the stripping effect is not ideal enough, and there is no Completely release the drug ingredients.
[0033] Table 1 Dissolution curve data of the product of the present invention
[...
Embodiment 3
[0037] Embodiment 3: Fluidity detection
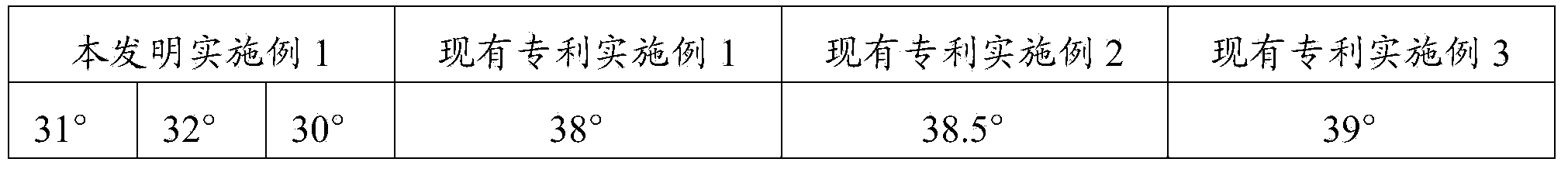
[0038] The fluidity of a solid cannot be expressed by a single characteristic value, and it is often expressed by the angle of repose. It usually refers to the largest angle formed by the free slope of the powder accumulation layer and the horizontal plane. The smaller the angle of repose, the smaller the friction, and the better the fluidity. It is generally believed that when θ≤30 degrees, the fluidity is good, and when θ≤40 degrees, it can meet the fluidity requirements in the production process. The fluidity of powder has a great influence on the weight difference and normal operation of granules, capsules, tablets and other preparations.
[0039] The composition of Example 1 was carried out to measure the angle of repose, which was repeated 3 times. At the same time, measure the angle of repose for the products prepared according to the method of CN1839838A embodiment 1-embodiment 3, each once.
[0040] Methods The injection me...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com