Method for preparing cupric tungstate via electrolysis of cation membrane
A technology of cationic membrane and copper tungstate, applied in the direction of electrolysis process, electrolysis components, organic diaphragm, etc., can solve the problems of reaction time of additives, etc., and achieve the effect of short reaction time, large synthesis amount and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
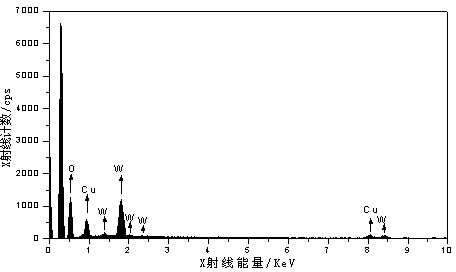
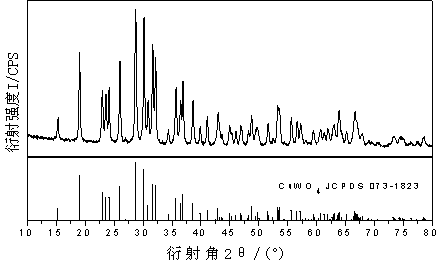
[0028] Using 95ml of 0.01mol / L sodium tungstate solution as anolyte, 90ml of 0.01mol / L sodium hydroxide solution as catholyte, copper sheet as anode and titanium mesh as cathode, electrolyze at a constant voltage of 50V for 10min. The electrolyzed product was washed three times with deionized water, and then dried in a blast drying oven at a constant temperature of 60°C for 2 hours. The temperature was raised to 500°C at a heating rate of 1 min, then roasted at a constant temperature for 1 h, and cooled to room temperature naturally. The obtained product is tested for EDS and XRD, such as figure 1 with figure 2 .
[0029] By observing figure 1 From the EDS diagram, it can be obtained that there are only three elements of Cu, W and O in the product. By observing figure 2 In the XRD pattern, it can be determined that the obtained product is CuWO 4 .
Embodiment 2
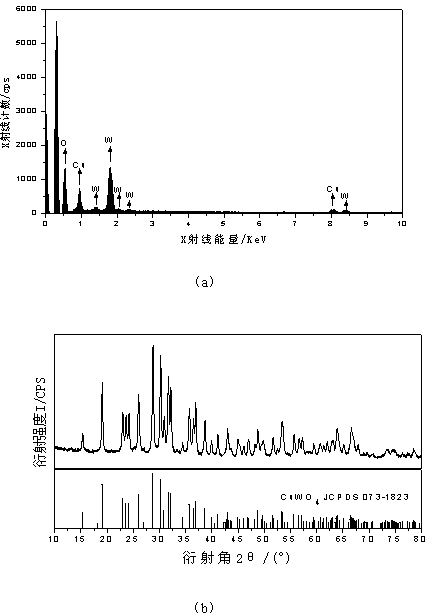
[0031] With 95ml of 0.01mol / L sodium tungstate solution as anolyte, 90ml of 0.01mol / L sodium hydroxide solution as catholyte, copper sheet as anode and titanium mesh as cathode, electrolyze 306S with a constant current of 0.6A (Electrolysis time is based on Na + The theoretical electricity consumed by all the ions transferred to the cathode chamber is calculated, that is, ). The electrolyzed product was washed three times with deionized water, and then dried in a blast drying oven at a constant temperature of 60°C for 2 hours. Raise the temperature to 500°C at a heating rate of min, then roast at constant temperature for 1h, and cool to room temperature naturally. The obtained product is tested for EDS and XRD, such as image 3 .
[0032] By observing image 3 From the EDS diagram of (a), it can be obtained that there are only three elements of Cu, W, and O in the product. By observing image 3 The XRD pattern in (b), it can be confirmed that the obtained product is Cu...
Embodiment 3
[0034] With 95ml of 0.01mol / L sodium tungstate solution as anolyte, 90ml of 0.01mol / L sodium chloride solution as catholyte, copper sheet as anode and titanium mesh as cathode, electrolyze 306S with a constant current of 0.6A . The electrolyzed product was washed three times with deionized water, and then dried in a blast drying oven at a constant temperature of 60°C for 2 hours. Raise the temperature to 500°C at a heating rate of min, then roast at constant temperature for 1h, and cool to room temperature naturally. The obtained product is tested for EDS and XRD, such as Figure 4 .
[0035] By observing Figure 4 From the EDS diagram of (a), it can be obtained that there are only three elements of Cu, W, and O in the product. By observing Figure 4 The XRD pattern in (b), it can be confirmed that the obtained product is CuWO 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com