Positron nuclide labeled selenocysteine compound and application thereof
A technology of selenocysteine and compound is applied in the application field of preparing positron emission tomography imaging agent, and the effect of good development prospect is achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
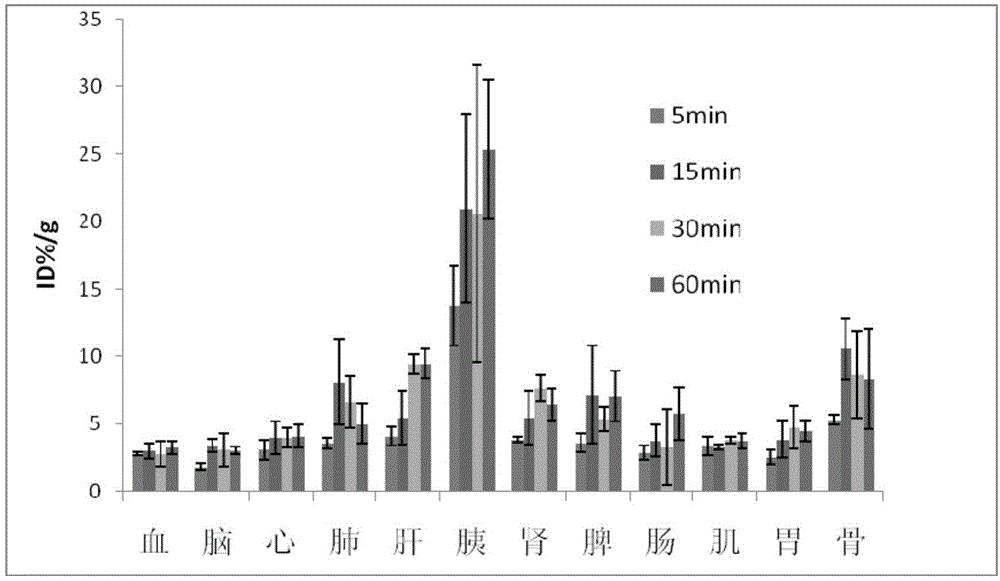
Image
Examples
Embodiment 111
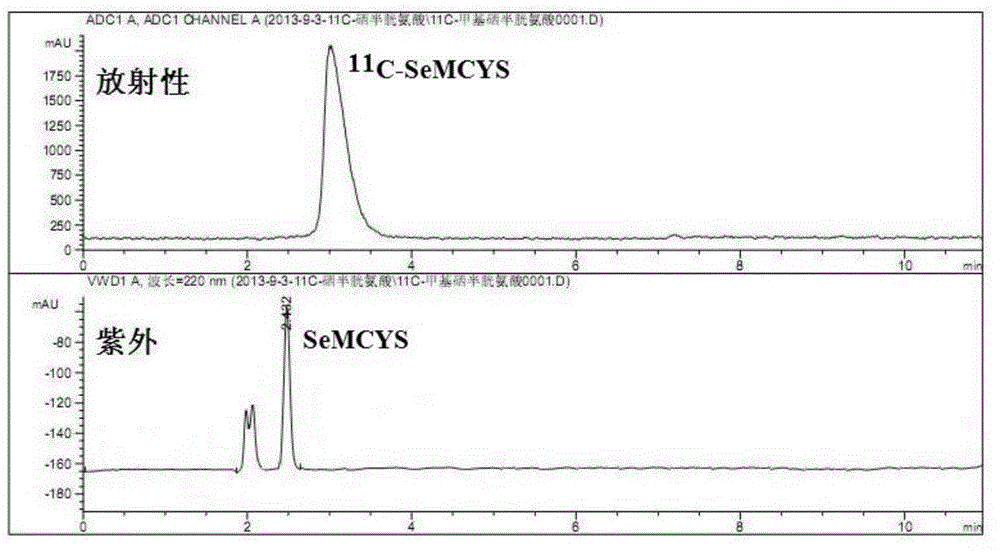
[0037] Example 1 11 Radiosynthesis of C-SeMCYS
[0038] 1 Materials and Instruments
[0039] 1.1 Main reagents and consumables. Kryptofix2.2.2 (K222), anhydrous acetonitrile, anhydrous K 2 CO 3 , lithium aluminum hydride, USP ethanol, 1.0N hydrochloric acid, dimethyl sulfoxide, and 1,3-propanediol-di-p-toluenesulfonate, products of Sigma-Aldrich Company in the United States; L-selenocysteine, domestic purchase; 18 O-H 2 O, ABX Company; Iodomethane, Sinopharm Chemical Reagent Co., Ltd.; Chloral hydrate, prepared by the First Affiliated Hospital of Sun Yat-sen University. Silica gel60TLC alumimium sheets5×10cm, Merck, Germany; Sep Pak Plus C18 column, Sep Pak light QMA column, Sep Pak plus SiO2 column, Sep Pak light CM column, Sep Pak Plus tC18 column, Water s, USA Product; Sep Pak SCX small column, product of Alltech Company in the United States.
[0040] 1.2 Main experimental instruments. Cyclone10 / 5 medical cyclotron, Belgian IBA company; PET-CS-II-IT-I carbon-11 met...
Embodiment 218F
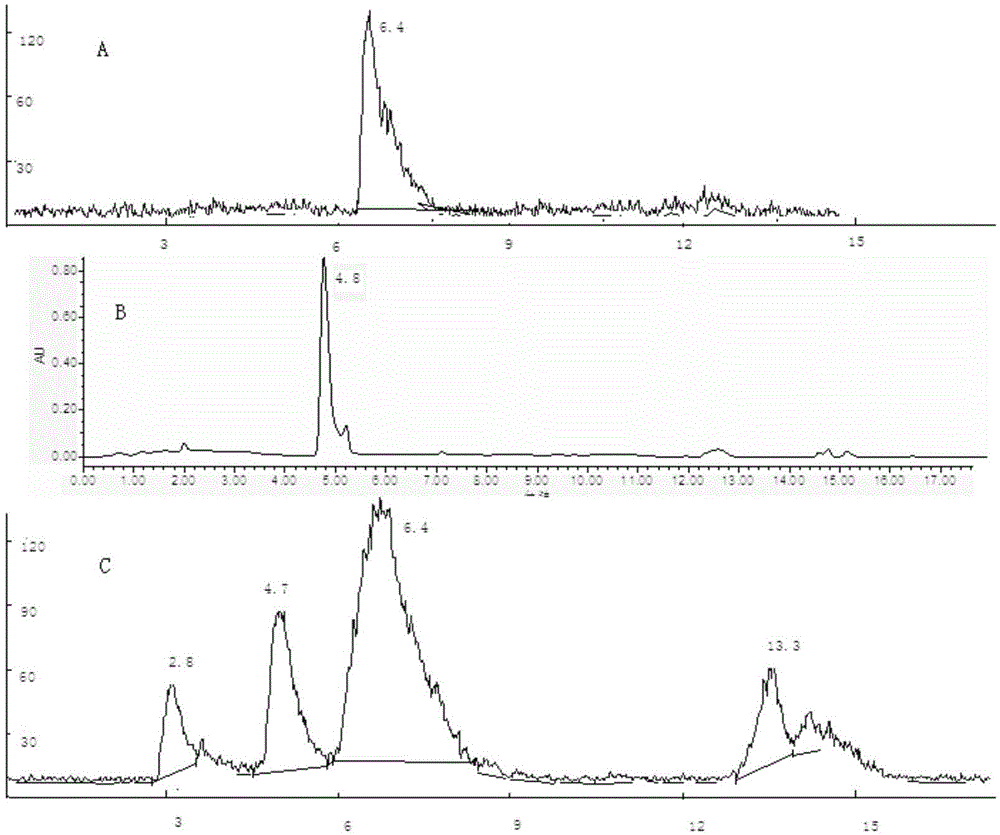
[0050] Example 2 18 F-labeled selenocysteine compounds 18 Synthesis of F-SeFPCYS
[0051] 2.1 18 F-labeled selenocysteine compounds 18 Synthesis of F-SeFPCYS
[0052] cyclotron through nuclear reaction 18 O(p,n) 18 F production gets 18 f - , after capture by QMA, with 1.0mL of K222 / K 2 CO 3 solution elution 18 f - into the reaction tube, feed N at 108°C 2 The solvent was evaporated to dryness. Then add 0.5 mL of anhydrous acetonitrile and evaporate to dryness, repeat three times. In the dry K222 solution, add propylene glycol 1,3-di-p-toluenesulfonate (TsOCH 2 CH 2 CH 2 OTs)7.7mg (0.02mmol) of anhydrous acetonitrile (1.0mL) solution, N 2 React at 90°C for 10 minutes under protection. After directly evaporating to dryness, a solution of 3.3 mg (0.02 mmol) of precursor L-selenocysteine dissolved in DMSO (0.5 mL) and 20 μL of 10% NaOH solution were added, and reacted at the same temperature for 5 min. Add 5mL of water, rinse the silica gel column with 5mL...
Embodiment 318
[0056] Example 3 18 Synthesis of F-SeFECYS
[0057] With the 1,3-dipropylene glycol tosylate (TsOCH 2 CH 2 CH 2 OTs) in anhydrous acetonitrile (1.0 mL) was replaced by 1,2-diethylene sulfonate (TsOCH 2 CH 2 OTs), all the other are with embodiment 2, make 18 F-SeFECYS Injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com