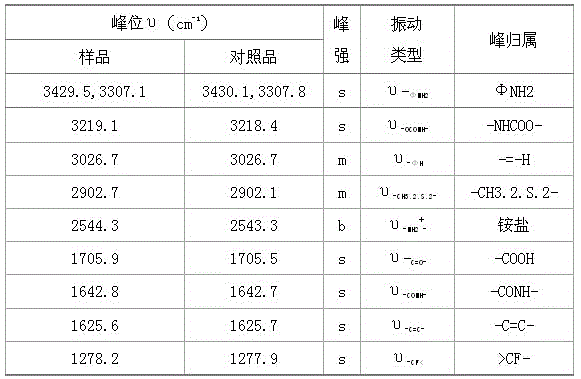
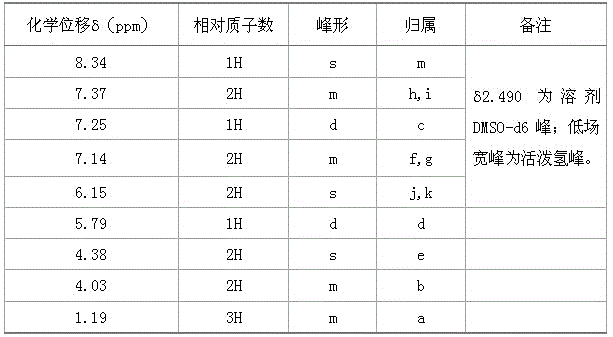
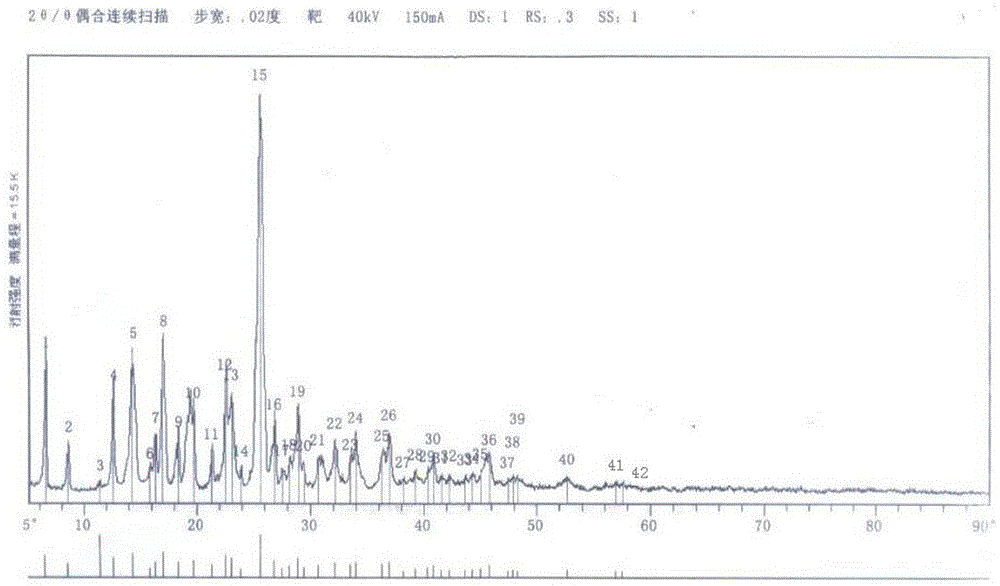
A kind of synthetic method of flupirtine maleate
A technology of flupirtine maleate and a synthesis method, applied in the field of medicine, can solve problems such as inability to stabilize pure A crystal form, and achieve the effects of white product appearance, stable yield and quality, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A kind of synthetic method of flupirtine maleate, its processing step is as follows:
[0035] A. Synthesis of 2-amino-3-nitro-6-(p-fluorobenzylamino)pyridine (I)
[0036] Add isopropanol and p-fluorobenzylamine into the reaction kettle, stir, add 2-amino-3-nitro-6-chloropyridine and triethylamine, reflux at 70°C for 12 hours, take samples to monitor the completion of the reaction, and then add hydrogen The sodium oxide solution was concentrated under reduced pressure until no solvent flowed out, centrifuged to obtain a solid, and dried to obtain 2-amino-3-nitro-6-(p-fluorobenzylamino)pyridine (I).
[0037]
[0038] B. Synthesis of ethyl 2-amino-3-carbamate-6-(p-fluorobenzylamino)pyridine (Ⅲ)
[0039] Add isopropanol, 2-amino-3-nitro-6-(p-fluorobenzylamino)pyridine (I) and Raney nickel into the autoclave, seal the autoclave, and feed nitrogen and hydrogen successively, at 60°C Carry out the hydrogenation reaction. After the hydrogenation reaction is completed, reduc...
Embodiment 2
[0045] A kind of synthetic method of flupirtine maleate, its processing step is as follows:
[0046] A. Synthesis of 2-amino-3-nitro-6-(p-fluorobenzylamino)pyridine
[0047] Add isopropanol and p-fluorobenzylamine into the reaction kettle, stir, add 2-amino-3-nitro-6-chloropyridine and triethylamine, reflux at 80°C for 8 hours, take samples to monitor the completion of the reaction, add hydrogen The sodium oxide solution was concentrated under reduced pressure until no solvent flowed out, centrifuged to obtain a solid, and dried to obtain 2-amino-3-nitro-6-(p-fluorobenzylamino)pyridine.
[0048] B. Synthesis of ethyl 2-amino-3-carbamate-6-(p-fluorobenzylamino)pyridine
[0049]Add isopropanol, 2-amino-3-nitro-6-(p-fluorobenzylamino)pyridine and Raney nickel into the autoclave, seal the autoclave, feed nitrogen and hydrogen in sequence, and carry out hydrogenation reaction at 70°C , after the hydrogenation reaction is completed, reduce the pressure to normal pressure, under th...
Embodiment 3
[0053] A kind of synthetic method of flupirtine maleate, its processing step is as follows:
[0054] A. Synthesis of 2-amino-3-nitro-6-(p-fluorobenzylamino)pyridine
[0055] Add isopropanol and p-fluorobenzylamine into the reaction kettle, stir, add 2-amino-3-nitro-6-chloropyridine and triethylamine, reflux at 75°C for 10 hours, take samples to monitor the completion of the reaction, and then add hydrogen The sodium oxide solution was concentrated under reduced pressure until no solvent flowed out, centrifuged to obtain a solid, and dried to obtain 2-amino-3-nitro-6-(p-fluorobenzylamino)pyridine.
[0056] B. Synthesis of ethyl 2-amino-3-carbamate-6-(p-fluorobenzylamino)pyridine
[0057] Add isopropanol, 2-amino-3-nitro-6-(p-fluorobenzylamino)pyridine and Raney nickel into the autoclave, close the autoclave, feed nitrogen and hydrogen in sequence, and carry out hydrogenation reaction at 65°C , after the hydrogenation reaction is completed, reduce the pressure to normal pressu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com