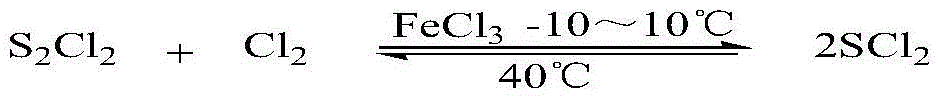Preparation method for synthetizing sulfur dichloride through gas phase catalysis
A gas-phase catalytic synthesis of sulfur dichloride and sulfur dichloride technology, applied in the direction of sulfur and halogen compounds, can solve the problems of small content, low content, large amount of three wastes, etc., achieve high conversion rate, reduce environmental pollution, operation simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: Preparation of supported ferric chloride granular activated carbon catalyst
[0026] Add 1000g FeCl to the reactor with reflux device 3 ·6H 2 O, dissolve it with 1200g of absolute ethanol, then add 80g of Φ2mm granular activated carbon, heat and reflux for 30min, cool, filter and dry in the air, place in a muffle furnace at 130-150°C and dry to constant weight to obtain a supported catalyst (FeCl 3 / C).
Embodiment 2
[0027] Embodiment 2: Preparation of sulfur dichloride by continuous gas phase method in fixed-bed catalytic tubular reactor
[0028] With the FeCl that makes in embodiment 1 3 / C 40g of granular catalyst is packed in a jacketed glass tubular reactor with an inner diameter of 20mm, the upper and lower ends of the catalyst are filled with magnetic rings, and the jacket is passed through hot water to raise the temperature to 40-50°C. Control the temperature of the vaporization chamber at 130°C, use chlorine gas as the carrier gas, add 67.5g of disulfur dichloride dropwise into the vaporization chamber, the molar ratio of chlorine gas:disulfur dichloride is 10:1, and control the flow rate of the mixed gas to 63.3ml / min . Chlorine and disulfur dichloride mixed gas are reacted through a fixed bed, and then cooled by a condenser to obtain 103.8 g of sulfur dichloride products, which contain 4.1 g of dissolved chlorine and 3.5 g of disulfur dichloride, which are transferred to storag...
Embodiment 3
[0029] Embodiment 3: Preparation of sulfur dichloride by continuous gas phase method in fixed-bed catalytic tubular reactor
[0030] With the FeCl that makes in embodiment 1 3 / C 40g of granular catalyst is loaded into a jacketed glass tube reactor with an inner diameter of 20mm, the upper and lower ends of the catalyst are filled with magnetic rings, and the jacket is passed through hot water to raise the temperature to 30-40°C. Control the temperature of the vaporization chamber at 130°C, use chlorine gas as the carrier gas, add 67.5g of disulfide dichloride dropwise into the vaporization chamber, the molar ratio of chlorine gas:disulfur dichloride is 9:1, and control the flow rate of the mixed gas to 53ml / min. Chlorine and disulfur dichloride mixed gas react by fixed bed, obtain 104.2g of sulfur dichloride product after condenser cooling again, wherein contain dissolved chlorine 3.8g, disulfur dichloride 2.5g, it is transferred to storage Low-temperature sealed storage in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com