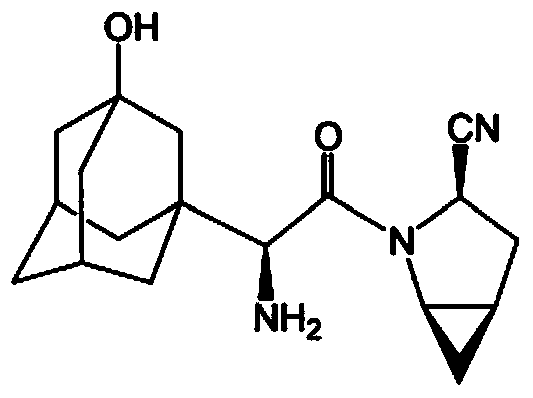
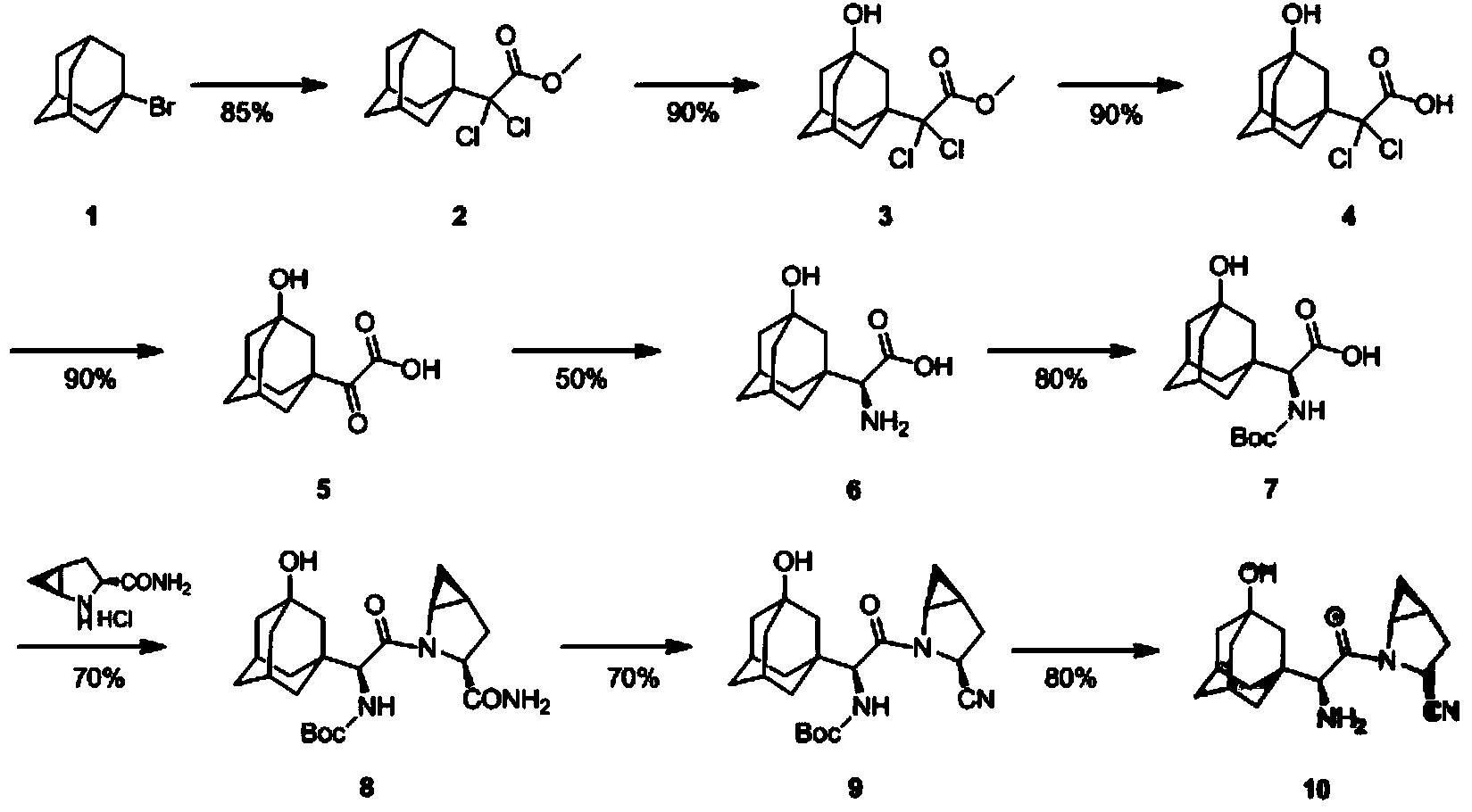
Method for preparing N-tert-butyloxycarbonyl-3-hydroxy-1-adamantyl-d-glycine
A technology of adamantylglycine and tert-butoxycarbonyl, which is applied in the field of preparation of N-tert-butoxycarbonyl-3-hydroxy-1-adamantylglycine, can solve the problems of difficult preparation and preservation of biological enzymes, and achieves Solve the effect of difficult preparation and preservation, lower production cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
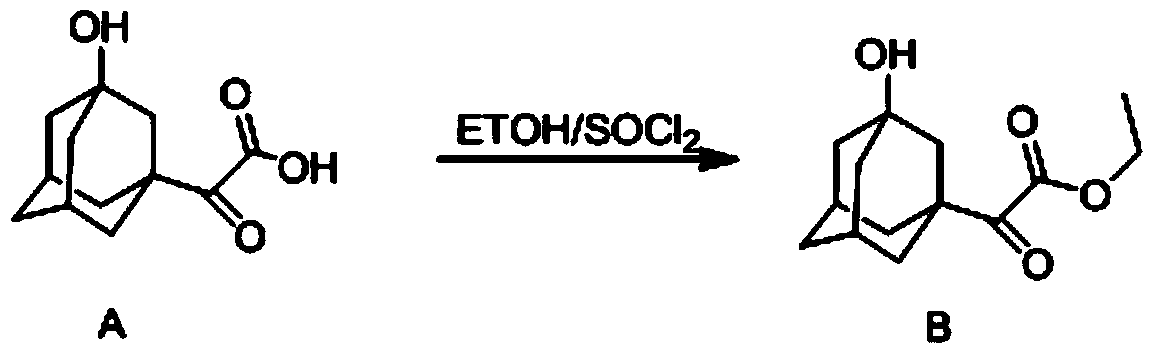
[0032] A preparation method of N-tert-butoxycarbonyl-3-hydroxyl-1-adamantylglycine, the compound 5[2-(3-hydroxyl-1-adamantyl)-2-1- The reaction process of oxoacetic acid (3)] to compound 7 (N-tert-butoxycarbonyl-3-hydroxyl-1-adamantylglycine) is carried out by replacing the reaction process of compound A to G in the following steps:
[0033](1) Put 130ml of absolute ethanol into a three-necked flask, cool it in an ice-salt bath to below -5°C, slowly add thionyl chloride (8ml, 110mmol) dropwise, and control the temperature not to exceed -5°C during the dropwise addition; After the addition was completed, compound A (20 g, 89 mmol) was added in batches, and the temperature was controlled to be lower than -5°C during the addition; after the addition was completed, the reaction solution was stirred at room temperature for 5 h, then the reaction solution was concentrated to 50 ml, and cooled to Below 0°C, add 100ml of toluene and 20g of triethylamine respectively; the reaction solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com