Purifying method for recombinant humanized anti-human tumor necrosis factor monoclonal antibody
A technology of tumor necrosis factor and monoclonal antibody, applied in the direction of microorganism-based methods, biochemical equipment and methods, anti-cytokine/lymphokine/interferon immunoglobulin, etc., can solve the problem that cannot meet the actual production requirements, antibody General effect of protein acidic components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
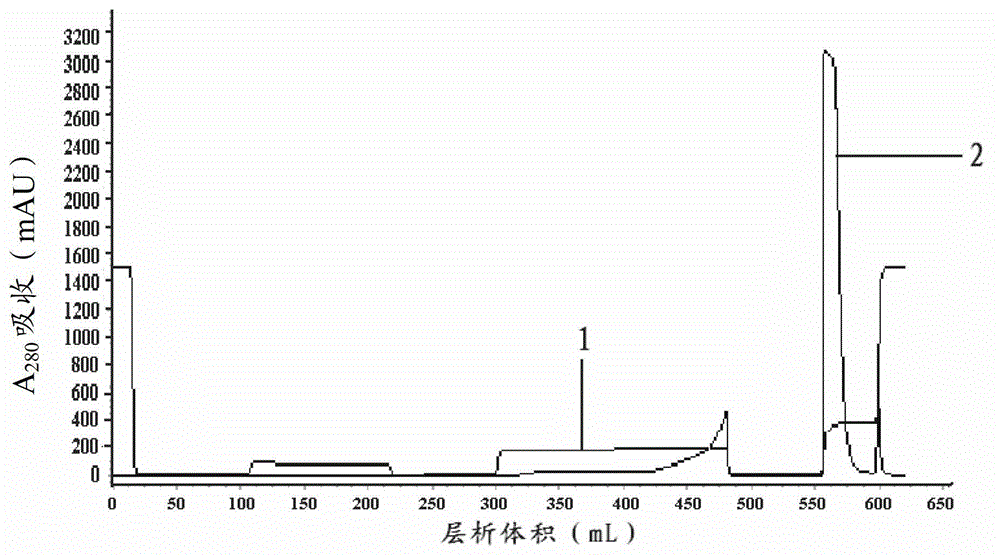
[0048] Example 1 Purification of recombinant human anti-human tumor necrosis factor monoclonal antibody using the method provided by the present invention
[0049] Use POROS XS to fill the cation exchange chromatography column;
[0050] The column is equilibrated with a phosphate buffer with a phosphate ion concentration of 50mmol / L and a pH value of 6.2;
[0051] The recombinant human anti-human tumor necrosis factor monoclonal antibody with a mass volume concentration of 27.3 mg / mL is loaded onto the equilibrated cation exchange chromatography column;
[0052] The concentration of phosphate ions is 50mmol / L, and the phosphate buffer solution with a pH value of 6.2 is used to wash the cation exchange chromatography column after loading the sample;
[0053] Use 12 times column volume of eluent to elute the washed cation exchange chromatography column, in the eluent:
[0054] Liquid A is a phosphate buffer with a concentration of phosphate ions of 50mmol / L, a pH value of 6.2,...
Embodiment 2
[0059] Example 2 Purification of recombinant human anti-human tumor necrosis factor monoclonal antibody using the method provided by the present invention
[0060] Use POROS XS to fill the cation exchange chromatography column;
[0061] The column is equilibrated with a phosphate buffer solution with a concentration of phosphate ions of 20mmol / L and a pH value of 5.8;
[0062] The recombinant human anti-human tumor necrosis factor monoclonal antibody with a mass volume concentration of 27.3 mg / mL is loaded onto the equilibrated cation exchange chromatography column;
[0063] The concentration of phosphate ions is 20mmol / L, and the phosphate buffer solution with a pH value of 5.8 is used to wash the cation exchange chromatography column after sample loading;
[0064] Use 12 times column volume of eluent to elute the washed cation exchange chromatography column, in the eluent:
[0065] Liquid A is a phosphate buffer solution with a concentration of phosphate ions of 20mmol / L, ...
Embodiment 3
[0070] Example 3 Purification of recombinant human anti-human tumor necrosis factor monoclonal antibody using the method provided by the present invention
[0071] Use POROS XS to fill the cation exchange chromatography column;
[0072] The column is equilibrated with a phosphate buffer solution with a concentration of phosphate ions of 80mmol / L and a pH value of 6.6;
[0073] The recombinant human anti-human tumor necrosis factor monoclonal antibody with a mass volume concentration of 27.3 mg / mL is loaded onto the equilibrated cation exchange chromatography column;
[0074] The concentration of phosphate ions is 80mmol / L, and the phosphate buffer solution with a pH value of 6.6 is used to wash the cation exchange chromatography column after loading the sample;
[0075] Use 12 times column volume of eluent to elute the washed cation exchange chromatography column, in the eluent:
[0076] Liquid A is a phosphate buffer solution with a concentration of phosphate ions of 80mmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com