Folic acid pharmaceutical composition for preventing administration
A composition and folic acid technology, applied in the field of medicine, can solve the problems of increasing impurity content and affecting the storage stability of preparations, and achieve the effects of reducing production time, reducing waste of human resources and improving output
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
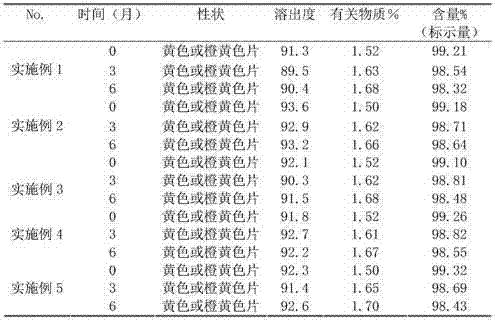
[0024] Example 1 Preparation of 0.4 mg / tablet folic acid tablets Take 10 g of folic acid, 2820 g of microcrystalline cellulose, 400 g of low-substituted hydroxypropyl cellulose, 150 g of methyl cellulose, 20 g of polyethylene glycol 6000, and 20 g of aspartame Pass through 100-200 mesh sieves respectively, take the above-mentioned components after sieving and mix evenly, dry granulate, pass through 24 mesh sieves, add 600020g of polyethylene glycol passed through 100-200 mesh sieves, mix evenly, measure the particle content, press Tablet, hardness 5~7kgf, pack, test, get finished product, get this method product and carry out disintegration experiment according to the requirement of two parts dispersible tablet of Chinese Pharmacopoeia 2010 edition, the product of present embodiment dissolves completely within 3 minutes in water.
Embodiment 2
[0025] Example 2 Preparation of 0.4 mg / tablet folic acid tablets Take 10 g of folic acid, 2900 g of microcrystalline cellulose, 500 g of low-substituted hydroxypropyl cellulose, 200 g of methyl cellulose, 40 g of polyethylene glycol 6000, and 200 g of β-cyclodextrin 1. Aspartame is 10g, passed through 100-200 mesh sieve respectively, take the above-mentioned components after sieving and mix evenly, dry granulate, pass through 20 mesh sieve, add 600020g of polyethylene glycol passed through 100-200 mesh sieve, Mix evenly, mix evenly, measure particle content, press tablet, hardness 5~7kgf, pack, test, get finished product, take the product of this method to carry out experiment according to the requirement of the second part dispersible tablet of Chinese Pharmacopoeia 2010 edition, the product of this embodiment is in water 3 Dissolves completely within minutes.
Embodiment 3
[0026] Example 3 Preparation of 0.4 mg / sheet folic acid tablets Take 10 g of folic acid, 2820 g of microcrystalline cellulose, 500 g of low-substituted hydroxypropyl cellulose, 100 g of polyvinylpyrrolidone K30, 20 g of polyethylene glycol, 200 g of β-cyclodextrin, 10g of aspartame is passed through a 100-200 mesh sieve, and the above-mentioned components after sieving are mixed evenly, dry granulated, passed through a 24-mesh sieve, and 600-20g of polyethylene glycol passed through a 100-200 mesh sieve is added, and mixed evenly , measure granule content, press tablet, hardness 5~7kgf, pack, test, get finished product, take the product of this method to carry out experiment according to the requirement of the second part dispersible tablet of Chinese Pharmacopoeia 2010 edition, the product of this embodiment dissolves completely in water within 3 minutes .
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com