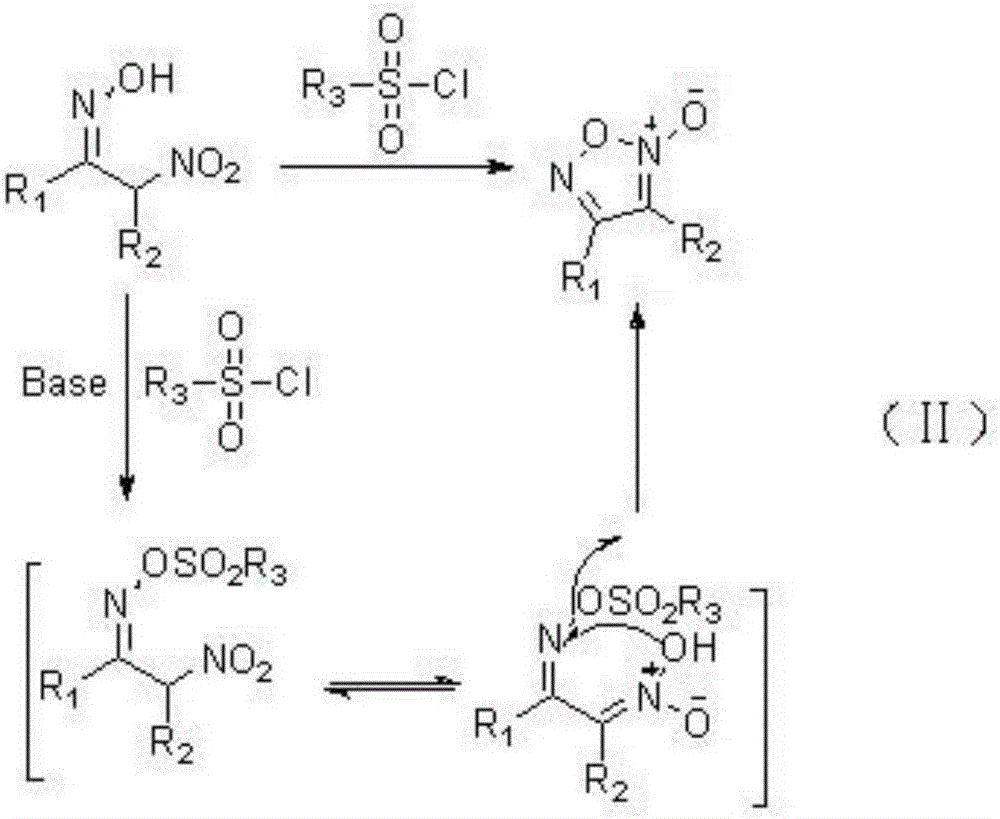
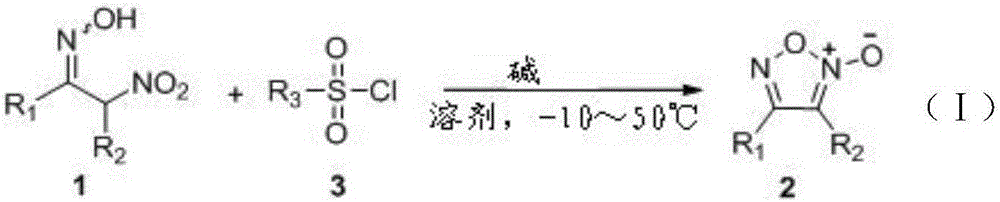A kind of method for synthesizing furoxan compound
A technology for oxidizing furoxan and compounds, which is applied in the field of synthesizing furoxan oxide compounds, can solve the problems of narrow application range, low yield and high cost, and achieves the effects of wide application scope, low price and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Synthesis of 3-methyl-4-phenylfuroxan
[0032] The synthesis method is: put a magnetic stirrer in a hard glass tube, weigh 38.8 mg (0.2 mmol) of 2-nitro-phenylpropyl-1-ketoxime and 47.6 mg (0.25 mg) of p-toluenesulfonyl chloride in sequence. mmol). Place the reaction tube under an ice bath, add 6 mL of mesitylene solvent, and finally add triethylenediamine hexahydrate (DABCO·6H 2 O) 44mg (0.2mmol), the reaction was continued to stir in ice bath for 20min, separated and purified to obtain 34mg of colorless crystals, yield 95%.
Embodiment 2
[0033] Example 2 Synthesis of 3-methyl-4-phenylfuroxan
[0034] The synthesis method is: put a magnetic stirrer in a hard glass tube, weigh 38.8 mg (0.2 mmol) of 2-nitro-phenylpropyl-1-ketoxime and 47.6 mg (0.25 mg) of p-toluenesulfonyl chloride in sequence. mmol). Place the reaction tube under an ice bath, add 6 mL of mesitylene solvent, and finally add 51.7 mg (0.4 mmol) of isopropylethylamine, and continue to stir the reaction at 50°C for 15 min. Separate and purify to obtain 28 mg of colorless crystals. rate of 68%.
Embodiment 3
[0035] Example 3 Synthesis of 3-methyl-4-phenylfuroxan
[0036] The synthesis method is: put a magnetic stirrer in a hard glass tube, weigh 38.8 mg (0.2 mmol) of 2-nitro-phenylpropyl-1-ketoxime and 76.16 mg (0.4 mg) of p-toluenesulfonyl chloride in sequence mmol). Place the reaction tube under an ice bath, add 6 mL of chloroform solvent, and finally add triethylenediamine hexahydrate (DABCO·6H 2 O) 44mg (0.2mmol), the reaction was continued to stir at 0°C for 20min, separated and purified to obtain 27mg of colorless crystals, with a yield of 75%.
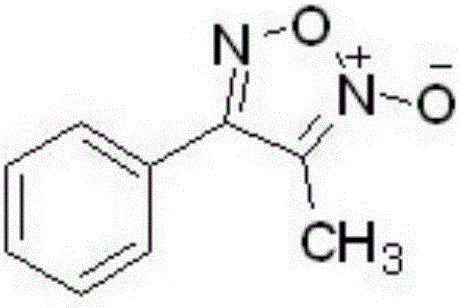
[0037] The colorless crystals obtained in Examples 1-3 have the same structural identification data, specifically as follows: m.p.94.3-95.1°C; 1 H NMR (300MHz, CDCl 3 ), δ(ppm):2.35(s,3H),7.52-7.55(m,3H),7.66-7.69(m,2H); 13 C NMR (75MHz, CDCl 3), δ (ppm): 9.1, 112.1, 126.7, 127.4, 129.2, 131.0, 156.8. HRMS (ESI) Calcd.for C 9 h 8 N 2 o 2 [M+Na] + :199.0478; found: 199.0483, it can be concluded that its structure is:
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com