Method for synthesizing neohesperidin dihydrochalcone from naringin
A technology for hesperidin dihydrochalcone and neohesperidin dihydrochalcone, which is applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve the problem of low yield and poor product purity , It is difficult to realize industrial production and other problems, and achieve the effect of high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
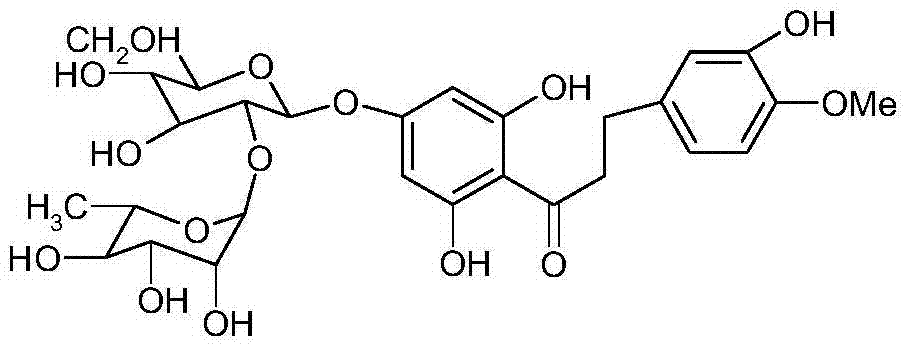
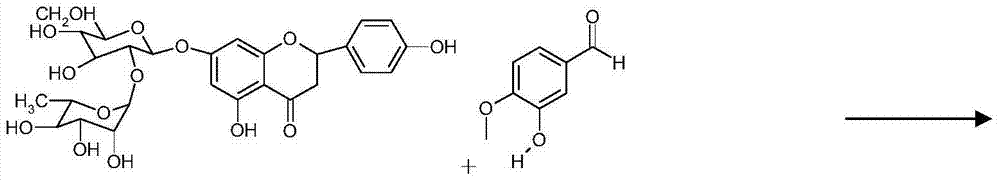
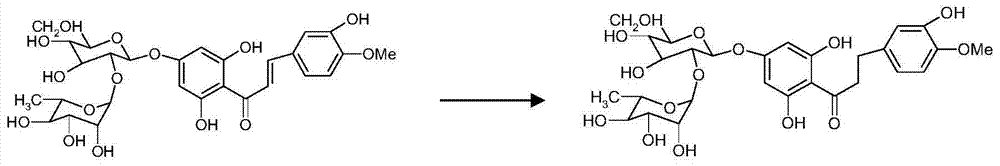
[0029] Raw material 95% naringin 100 grams, add 2000ml mass concentration and be in the potassium hydroxide solution of 20%, be heated to 80 ℃, then add the isovanillin of 300 grams in batches (half an hour 100 grams, add in three batches), Add the last batch and start timing, boil and reflux for 4.5h, cool, add 500ml of dichloromethane and stir and extract three times, the dichloromethane layer is concentrated to dryness, it is isovanillin, recovered for later use, add 2 grams of 10% Raney to the lye Nickel catalyst, hydrogenation temperature 30-40°C, pressure 1.0MP, reaction 8h. Suction filter the reaction solution to remove the catalyst, neutralize the filtrate with concentrated hydrochloric acid in an ice bath, place it for 8 hours, and filter it with suction, the solid is the crude neohesperidin dihydrochalcone, dissolve it in 3 times the amount of hot water at 80°C, and place it at 0°C 8h, suction filtration, and treated twice according to the above method to obtain 30 g...
example 2
[0031] Raw material 98% naringin 100 grams, add 2000ml mass concentration and be in the potassium hydroxide solution of 20%, be heated to 80 ℃, then add the isovanillin of 400 grams in batches (half an hour 133 grams, add in three batches), Add the last batch and start timing, boil and reflux for 4.5 hours, cool, add 500ml of dichloromethane and stir and extract three times, the dichloromethane layer is concentrated to dryness, and it is isovanillin, which is recovered for later use, and 1 g of 1% palladium carbon is added to the lye Catalyst, hydrogenation temperature 30-40°C, pressure 1.0MP, reaction 8h. Suction filter the reaction solution to remove the catalyst, neutralize the filtrate with concentrated hydrochloric acid in an ice bath, place it for 8 hours, and filter it with suction, the solid is the crude neohesperidin dihydrochalcone, dissolve it in 3 times the amount of hot water at 80°C, and place it at 0°C 8h, suction filtration, and treated twice according to the a...
example 3
[0033] Raw material 98% naringin 100 grams, add 2000ml mass concentration and be in the potassium hydroxide solution of 20%, be heated to 80 ℃, then add the isovanillin of 500 grams in batches (half an hour 167 grams, add in three batches), Add the last batch and start timing, boil and reflux for 4.5 hours, cool, add 500ml of dichloromethane and stir and extract three times, the dichloromethane layer is concentrated to dryness, and it is isovanillin, which is recovered for later use, and 1 g of 1% palladium carbon is added to the lye Catalyst, hydrogenation temperature 30-40°C, pressure 1.0MP, reaction 8h. Suction filter the reaction solution to remove the catalyst, neutralize the filtrate with concentrated hydrochloric acid in an ice bath, place it for 8 hours, and filter it with suction. The solid is the crude neohesperidin dihydrochalcone. Dissolve it in 5 times the amount of hot water at 85°C, Put it aside for 8 hours, filter it with suction, and process it twice according...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com