Method and kit for detecting mutation of PDS gene IVS7-2A>G by using fluorescence PCR technology
A kit and gene technology, used in fluorescence/phosphorescence, biochemical equipment and methods, and microbial determination/inspection, etc., can solve the problems of many steps, difficult to promote, and unsuitable for clinical promotion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: PDS gene IVS7-2A>G mutation detection kit and its use
[0038] Composition of the kit
[0039] 1 tube of PCR reaction solution (500ul), 1 tube of positive quality control product (20ul), 1 tube of negative quality control product (20ul), 1 tube of DNA extraction solution (3ml), 1 bottle of red blood cell lysate (15ml).
[0040] The PCR reaction solution includes: 1XPCR Buffer, 0.2mM dNTPs, Taq enzyme 25U / ml, 0.2uM forward primer (SEQ ID NO: 1), 0.2uM reverse primer (SEQ ID NO: 2), 0.2uM wild-type fluorescent probe Needle (SEQ ID NO: 3), 0.2 uM mutant fluorescent probe (SEQ ID NO: 4).
[0041] Steps:
[0042] 1) Gene extraction: Take 100ul EDTA anticoagulated peripheral blood, add 600ul red blood cell lysate, place at room temperature for 5-10 minutes, mix several times during this period, centrifuge at 5000rpm for 5 minutes, discard the supernatant, then add 100ul DNA lysate, mix well , 99 degrees for 10 minutes, centrifuged, and the supernatant was taken...
Embodiment 2
[0048] Example 2: PDS gene IVS7-2A>G mutation detection kit and its use
[0049] Composition of the kit
[0050] 1 tube of PCR reaction solution (500ul), 1 tube of positive quality control product (20ul), 1 tube of negative quality control product (20ul), 1 tube of DNA extraction solution (3ml), 1 bottle of red blood cell lysate (15ml).
[0051] The PCR reaction solution includes: 1XPCR Buffer, 0.2mM dNTPs, Taq enzyme 25U / ml, 0.2uM forward primer (SEQ ID NO: 1), 0.2uM reverse primer (SEQ ID NO: 2), 0.2uM mutant fluorescent probe needle (SEQ ID NO: 4).
[0052] The operating steps are the same as in Example 1.
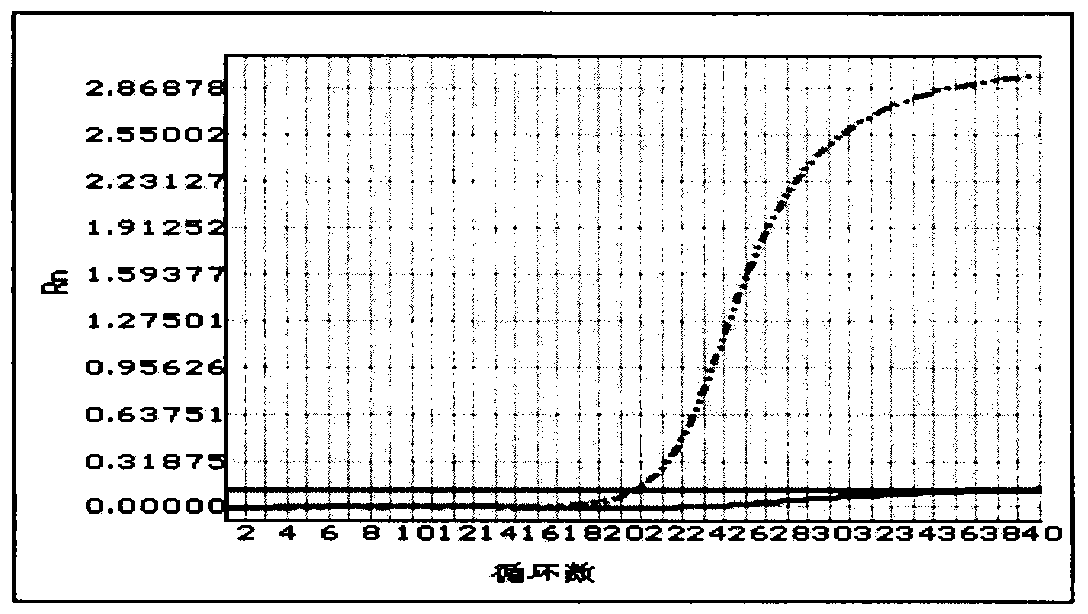
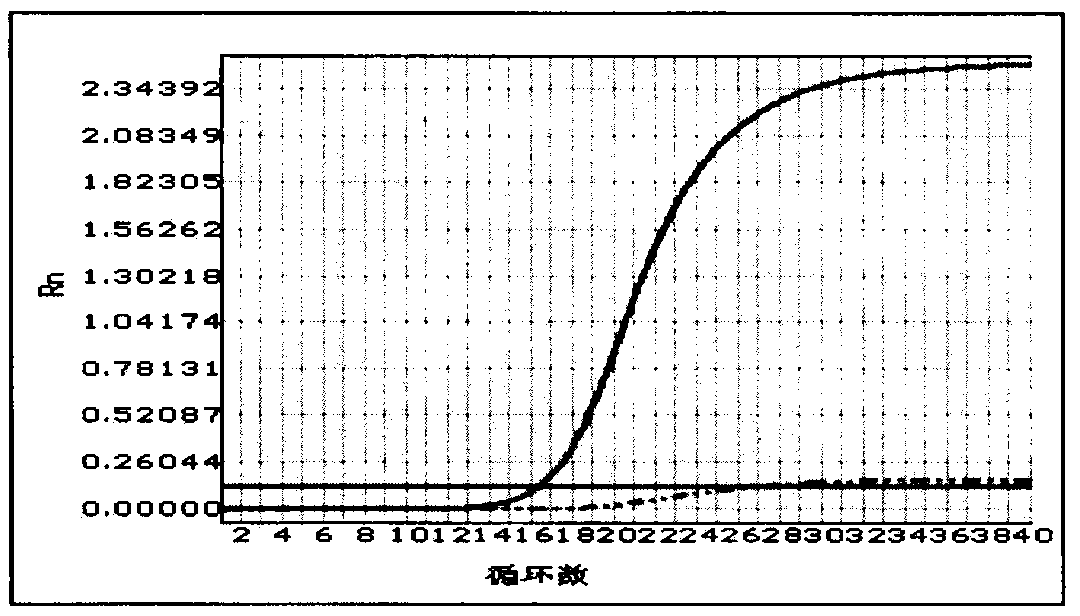
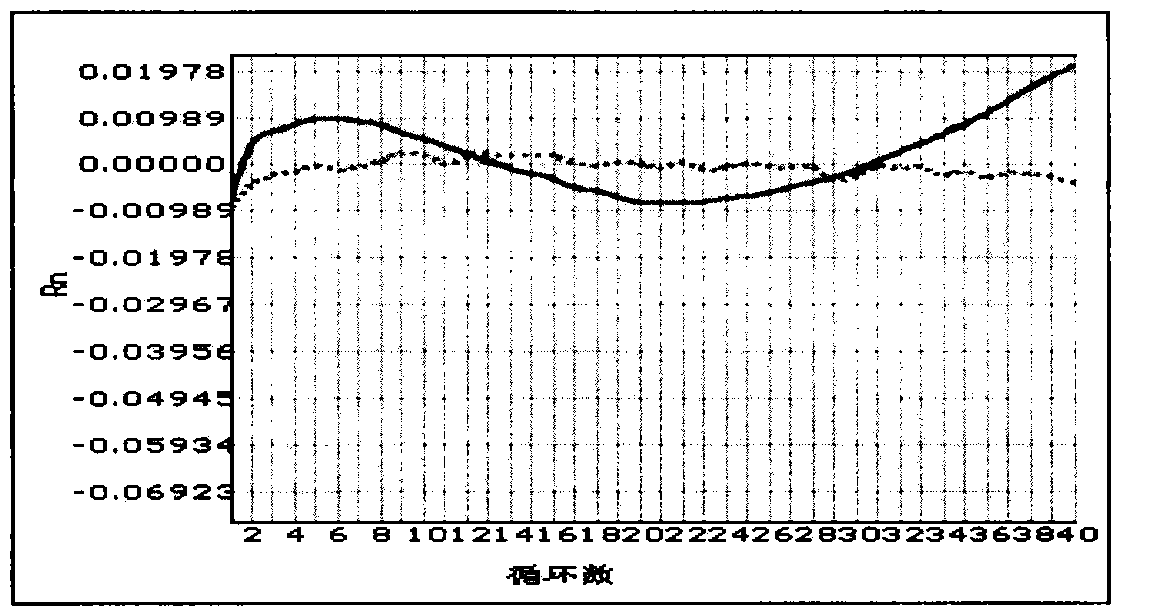
[0053] Result analysis: Analyze the samples according to the fluorescence color curve of the positive quality control product. If there is a curve with the same fluorescent color as the positive quality control product, the sample has the PDS gene IVS7-2A>G mutation, and it is impossible to distinguish heterozygous or homozygous mutations. If there is no fluorescenc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com