Method for continuously producing propylene oxide by directly oxidizing propylene with hydrogen peroxide
A technology of propylene oxide and hydrogen peroxide, applied in chemical recycling, organic chemistry and other directions, can solve the problems of a large amount of waste water and waste residue, high equipment cost, high process operation pressure, etc., to reduce load, maintain continuity, and reduce energy consumption. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
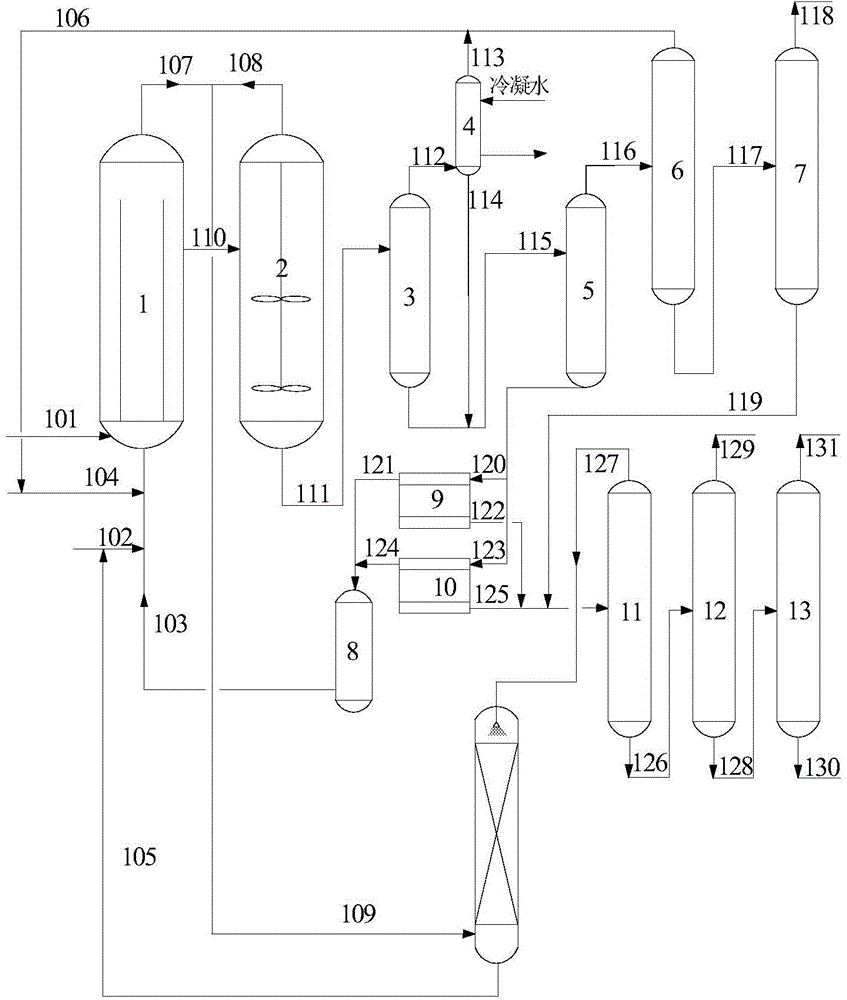
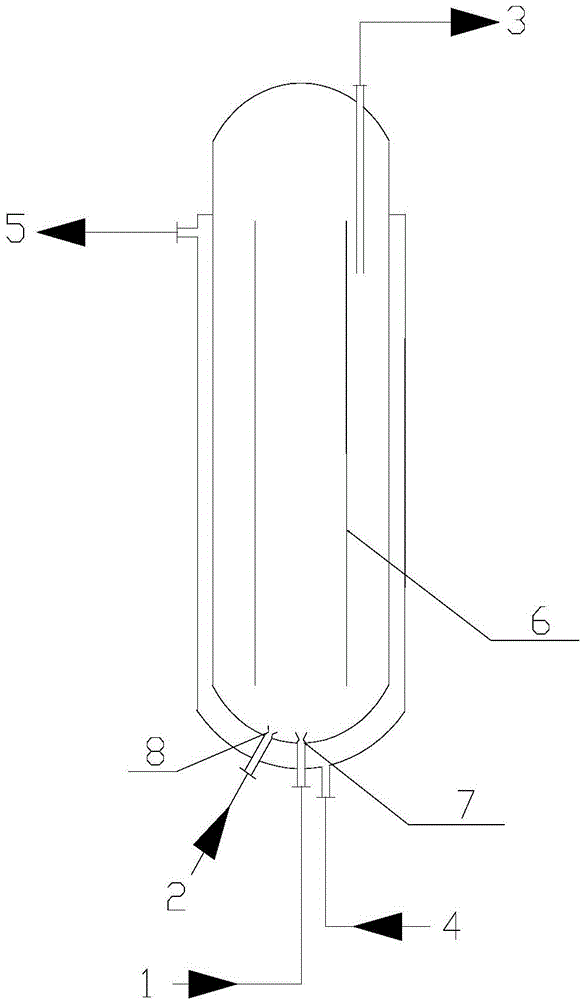
[0023] Such as figure 1 Shown flow process, the massfraction of the technical grade propylene of 500Kg / h, 981.2Kg / h is 27.5% hydrogen peroxide aqueous solution and solvent methyl alcohol, enter fluidized bed circulation main reactor 1 through pipeline 104,101 and 102 respectively, catalyst storage The catalyst in the tank 8 enters the main fluidized bed circulation reactor 1 at a flow rate of 50Kg / h, wherein the molar ratio of propylene:hydrogen peroxide:methanol is 1.5:1:16, the fluidized bed circulation main reactor 1 and the slurry stirring secondary reactor 2 The reaction temperature is 20°C, the reaction pressure is maintained at 0.6MPa, the stirring rate of the slurry stirring secondary reactor 2 is 80rpm, and the unreacted oxygen-containing material at the top of the fluidized bed circulation main reactor 1 and the slurry stirring secondary reactor 2 Propylene is merged in the pipeline 109, and the recycled methanol solvent is used to spray and absorb oxygen-containing ...
example 2
[0025] Such as figure 1 Shown flow process, the massfraction of the technical grade propylene of 500Kg / h, 1226.6Kg / h is 27.5% hydrogen peroxide aqueous solution and solvent methyl alcohol, enter fluidized bed circulation main reactor 1 through pipeline 104,101 and 102 respectively, catalyst storage The catalyst in the tank 8 enters the fluidized bed circulation main reactor 1 at a flow rate of 50Kg / h, wherein the molar ratio of propylene:hydrogen peroxide:methanol is 1.2:1:8, the fluidized bed circulation main reactor 1 and the slurry stirring secondary reactor 2 The reaction temperature is 45 ℃, the reaction pressure is maintained at 0.1MPa, the stirring rate of the slurry stirring secondary reactor 2 is 60rpm, and the unreacted oxygen-containing gas at the top of the fluidized bed circulation main reactor 1 and the slurry stirring secondary reactor 2 Propylene is merged in the pipeline 109, and the recovered methanol solvent is used to absorb oxypropylene in the bubbling abs...
example 3
[0027] Such as figure 1 Shown flow process, the massfraction of the technical grade propylene of 500Kg / h, 1468.3Kg / h is 27.5% hydrogen peroxide aqueous solution and solvent methyl alcohol, enter fluidized bed circulation main reactor 1 through pipeline 104,101 and 102 respectively, catalyst storage The catalyst in the tank 8 enters the fluidized bed circulation main reactor 1 at a flow rate of 50Kg / h, wherein the molar ratio of propylene:hydrogen peroxide:methanol is 1.0:1:24, the fluidized bed circulation main reactor 1 and the slurry stirring secondary reactor 2 The reaction temperature is 50°C, the reaction pressure is maintained at 1.0MPa, the stirring rate of the slurry stirring secondary reactor 2 is 100rpm, and the unreacted oxygen-containing gas at the top of the fluidized bed circulation main reactor 1 and the slurry stirring secondary reactor 2 Propylene is merged in the pipeline 109, and after absorbing oxygen-containing propylene with the recovered methanol solvent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com