R-lansoprazole methylamine salt compound and preparation method and pharmaceutical composition thereof
A technology of lansoprazole methylamine and compounds, applied in the field of drug synthesis, capable of solving problems such as poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of dexlansoprazole methylamine salt compound:
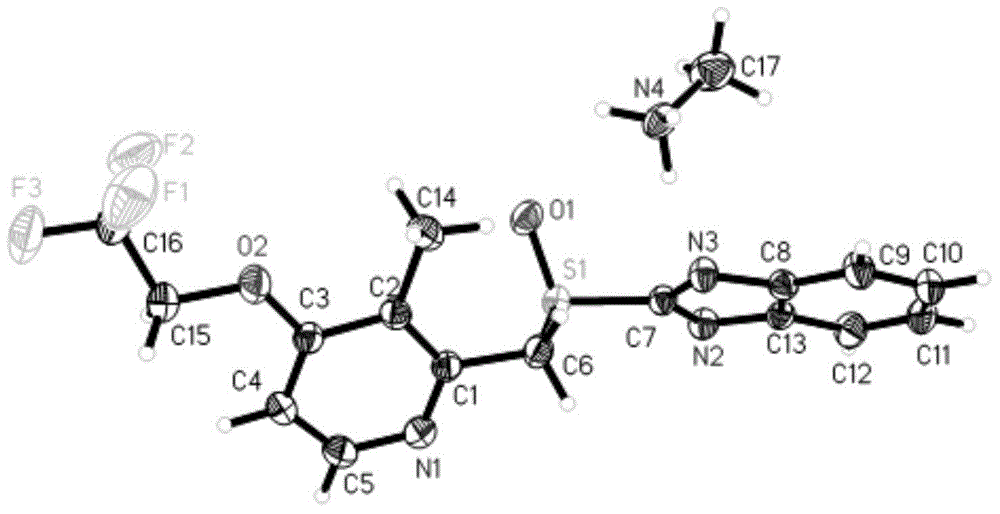
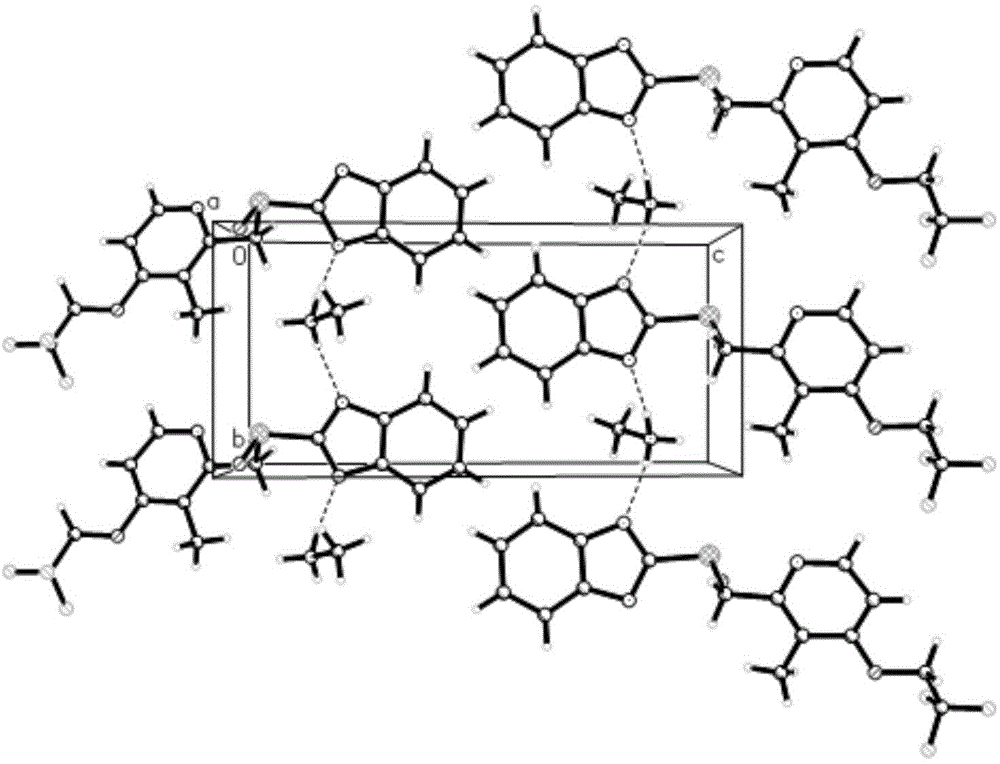
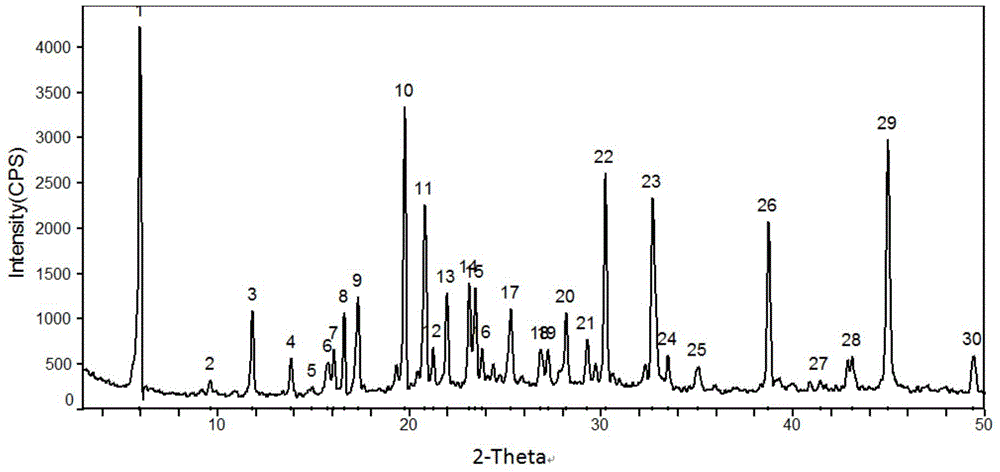
[0045] 5g of dexlansoprazole was dissolved in 50mL of methanol solution saturated with methylamine gas in advance, and heated at 40°C to slightly reflux. In order to increase the yield and prevent methylamine from escaping, a closed system can also be used for the reaction until The reaction was complete; the temperature of the reaction solution was slowly cooled to room temperature, and then to 0° C., white needle-shaped crystals were precipitated, filtered, and dried to obtain 4.2 g of white needle-shaped crystals with a water content of 0.16%. The obtained needle-like crystals are analyzed to obtain the corresponding X-ray powder diffraction pattern, DSC spectrum and TG spectrum, respectively as image 3 , Figure 4 with Figure 5 shown.
[0046] Get the above-mentioned product and measure proton nuclear magnetic spectrum, get as follows Image 6 The hydrogen spectrum shown, 1 H-NMR (400 MHz, DMSO):...
Embodiment 2
[0051] Dissolve 5g of dexlansoprazole in a mixed solution of 25mL of ethylene glycol and 3.5g of 25% methylamine aqueous solution, heat at 50°C to slightly reflux until the reaction is complete; the reaction solution is cooled to about 10°C, and white needle-like crystals are precipitated, filtered , washed with water to remove ethylene glycol, and dried to obtain 4.92 g of white crystals with a water content of 0.30%. The obtained crystals were analyzed by H NMR spectrum to obtain the same Image 6 Consistent results are shown for the dexlansoprazole methylamine salt compound.
[0052] Changing the solvent in the above Example 2 to acetonitrile or N,N-dimethylformamide also obtained the dexlansoprazole methylamine salt compound consistent with the above.
Embodiment 3
[0054] Suspend 5g of dexlansoprazole in a two-phase mixed solution of 50mL of toluene and 5g of 25% methylamine aqueous solution, seal the reaction and stir vigorously at 30°C for 2 days to complete the reaction; cool the reaction solution to 5°C, filter the resulting white fine needles crystallized, and dried to obtain 5.05 g of white crystalline powder with a water content of 0.56%. The obtained crystals were analyzed by H NMR spectrum to obtain the same Image 6 Consistent results are shown for the dexlansoprazole methylamine salt compound.
[0055] The solvent in above-mentioned embodiment 3 is changed into ethyl acetate, also obtained the dexlansoprazole methylamine salt compound consistent with above-mentioned.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com