Pyrimidylsalicylate type compounds, and preparing method and applications thereof
A technology for pyrimidine salicylic acids and compounds, which is applied in the field of pyrimidine salicylic acids and their preparation, and achieves the effect of effective prevention and treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
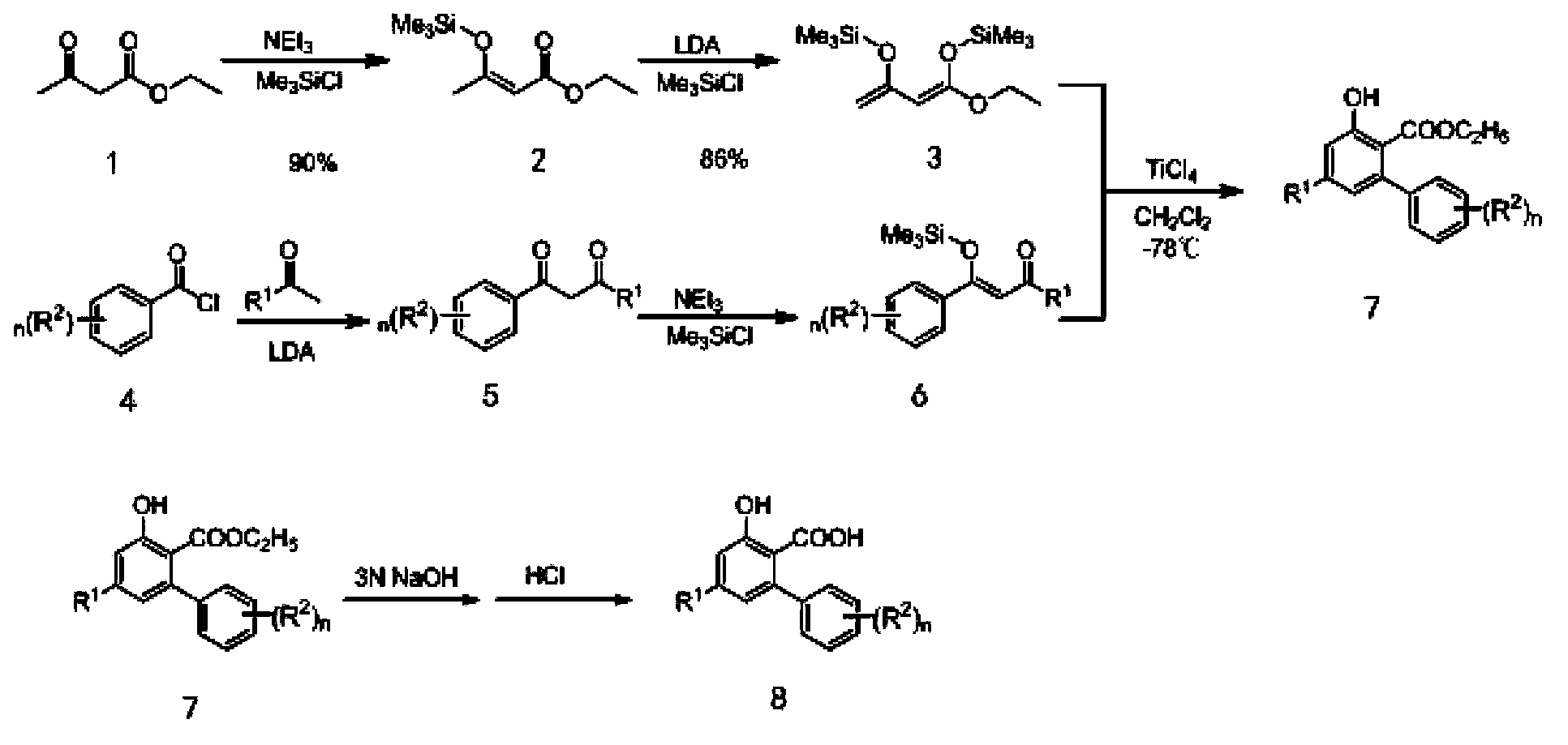
[0040] In a second aspect, the present invention provides a method for preparing pyrimidine salicylic acid compounds represented by formula (I), the method comprising the following steps:
[0041] (1) Dissolving the compound represented by formula (II) in an organic solvent, and then contacting it with a basic inorganic salt, so that the hydrogen on the hydroxyl group in the compound of formula (II) is replaced;
[0042] (2) The product obtained in step (1) is contacted with the pyrimidine compound represented by formula (III) to carry out nucleophilic substitution reaction, and the aqueous solution of the solid product obtained by the reaction is acidified to a pH of 0-1 to obtain formula (I) The indicated pyrimidine salicylates;
[0043] Formula (I)
[0044] Formula (II)
[0045] Formula (III)
[0046] in,
[0047] R 1 is methyl or trifluoromethyl;
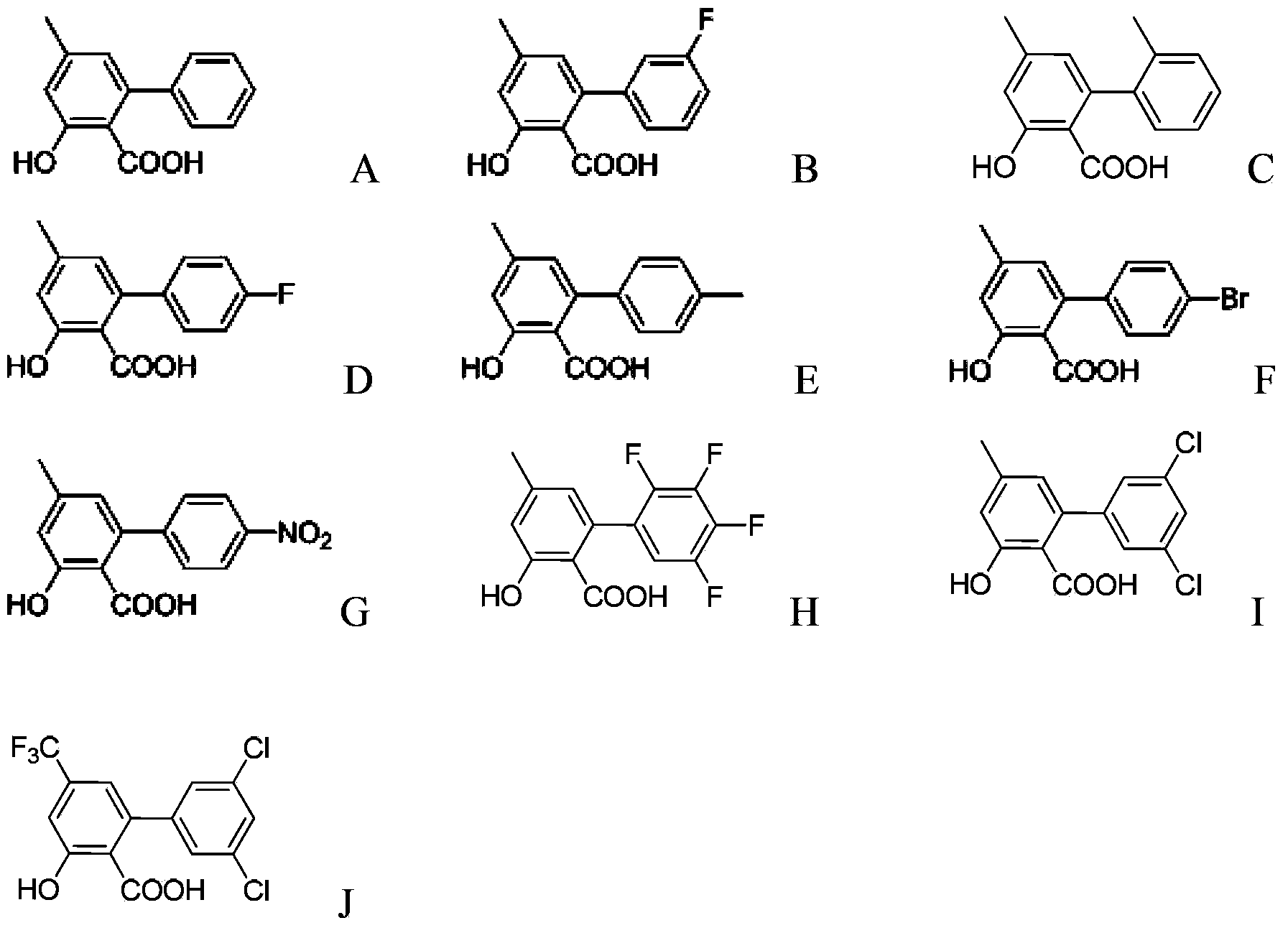
[0048] R 2 At least one selected from hydrogen, methyl, halogen, nitro, methoxy and trifluoromethyl, n is 0, 1, 2...
Embodiment 1
[0075] This example is used to illustrate the pyrimidine salicylate compound of the present invention and its preparation method.
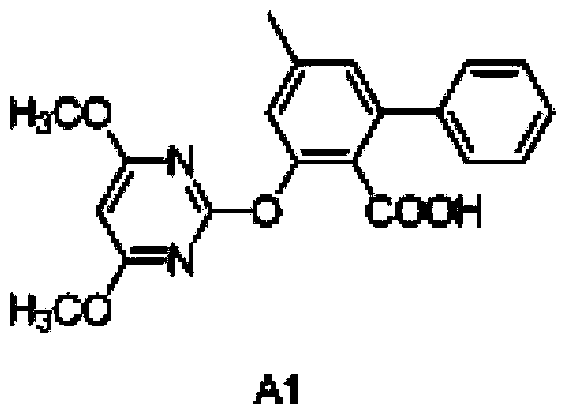
[0076] Dissolve 1mmol of compound A in 30mL of redistilled toluene, add 2mmol of potassium carbonate, react at 20°C for 2 hours, then add 4,6-dimethoxy-2-thiamphenicol pyrimidine (1mmol), at 120°C Under reaction 12h. The solvent was distilled off under reduced pressure, a small amount of water was added to dissolve the solid completely, the aqueous layer was washed several times with ether, the aqueous layer was acidified to pH=1 with concentrated hydrochloric acid, extracted with dichloromethane, dried over anhydrous sodium sulfate, and pyrimidine salicyl was obtained by column chromatography Acid compound A1. The yield of Compound A1 prepared from Compound A was 78%. A1(Y11149):mp:144-145℃. 1 H NMR(600MHz,dmso)δ12.83(s,1H),7.45–7.40(m,4H),7.38(d,J=6.6Hz,1H),7.15(s,1H),7.13(s,1H) ,6.00(s,1H),3.79(s,6H),2.39(s,3H).HRMS(MALDI):Calcd for C 20 h...
Embodiment 2
[0079] This example is used to illustrate the pyrimidine salicylate compound of the present invention and its preparation method.
[0080] Dissolve 1mmol of compound B in 30mL of redistilled toluene, add 2mmol of potassium carbonate, react at 23°C for 1.5 hours, then add 4,6-dimethoxy-2-thiamphenicol pyrimidine (1mmol), at 118°C Under reaction 16h. The solvent was distilled off under reduced pressure, a small amount of water was added to dissolve the solid completely, the aqueous layer was washed several times with ether, the aqueous layer was acidified to pH=1 with concentrated hydrochloric acid, extracted with dichloromethane, dried over anhydrous sodium sulfate, and pyrimidine salicyl was obtained by column chromatography Acid compound B1. The yield of Compound B1 prepared from Compound B was 65%. B1(Y11150):mp:140-141℃. 1 H NMR(600MHz,dmso)δ12.99(s,1H),7.48(d,J=7.2Hz,1H),7.33–7.08(m,5H),6.02(s,1H),3.79(s,6H) ,2.39(s,3H).HRMS(MALDI):Calcd for C 20 h 17 FN 2 o 5 [M+H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com