Red organic electrophosphorescent material iridium metal complex, preparation method thereof, and organic electroluminescent device
A technology of iridium metal complexes and phosphorescent materials, applied in the field of organic electroluminescence, which can solve the problems of backwardness and rarely achieve the color purity of dark red and dark green light, and achieve high luminous efficiency and good energy transmission efficiency , good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
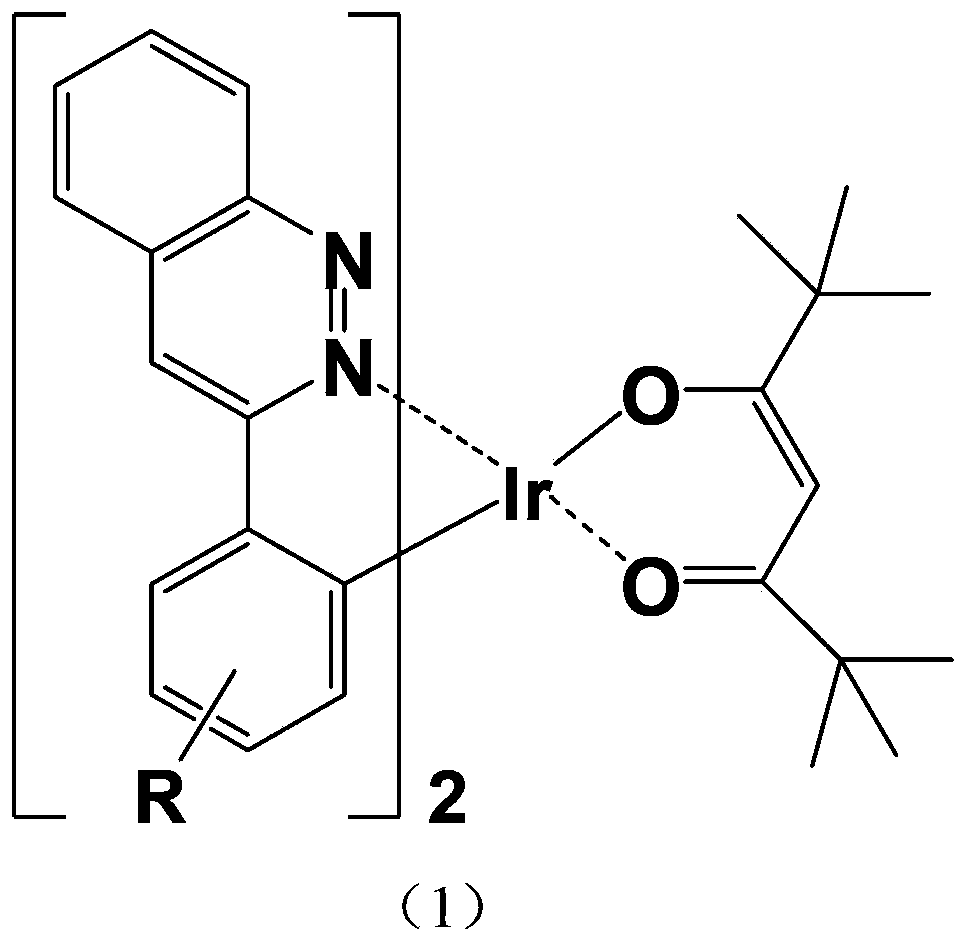
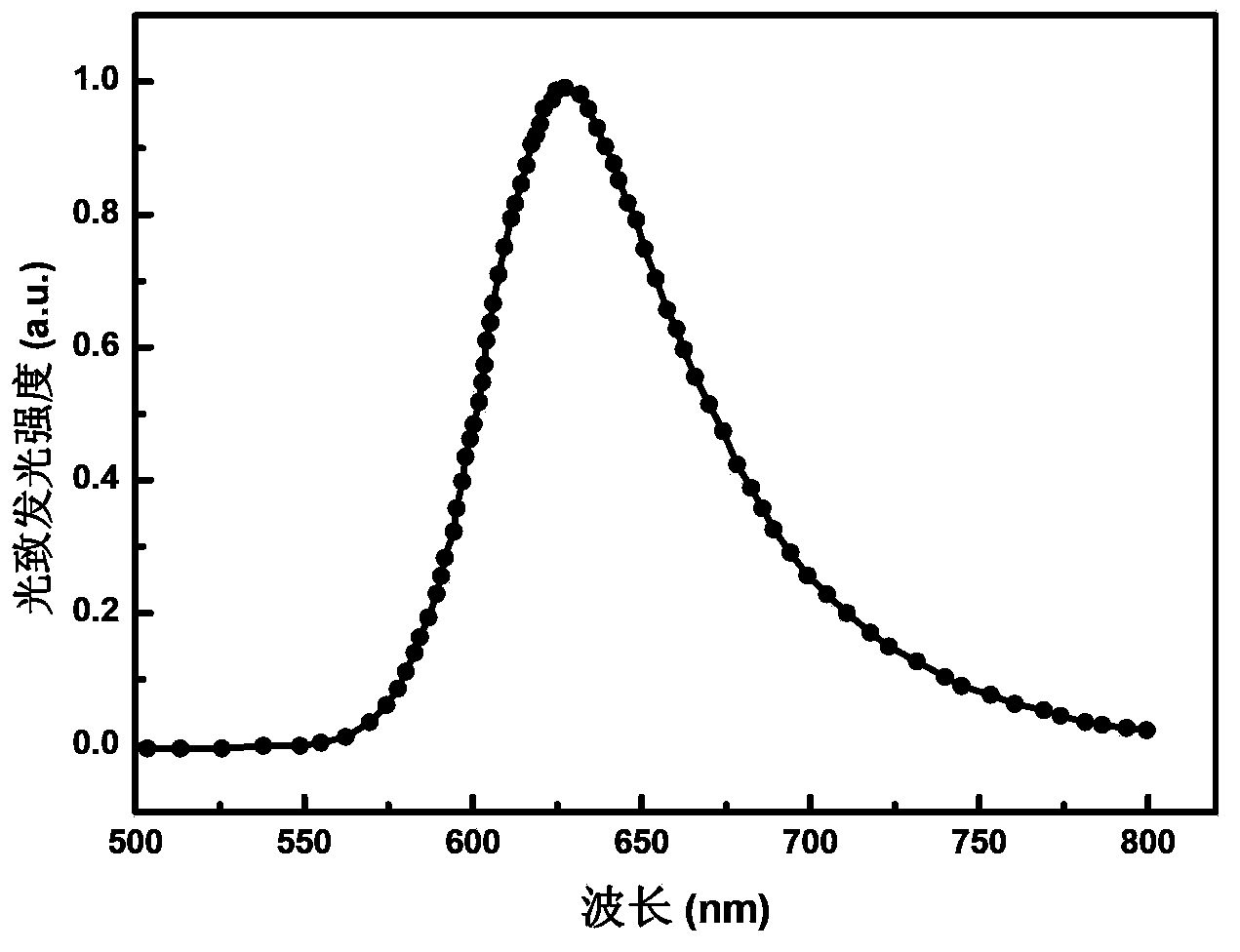
[0064] Example 1: A red-light organic electrophosphorescent material iridium metal complex bis[3-phenylcinnoline-N,C 2 '](2,2,6,6-tetramethyl-3,5-heptanedione) iridium, as shown in the following structural formula:
[0065]
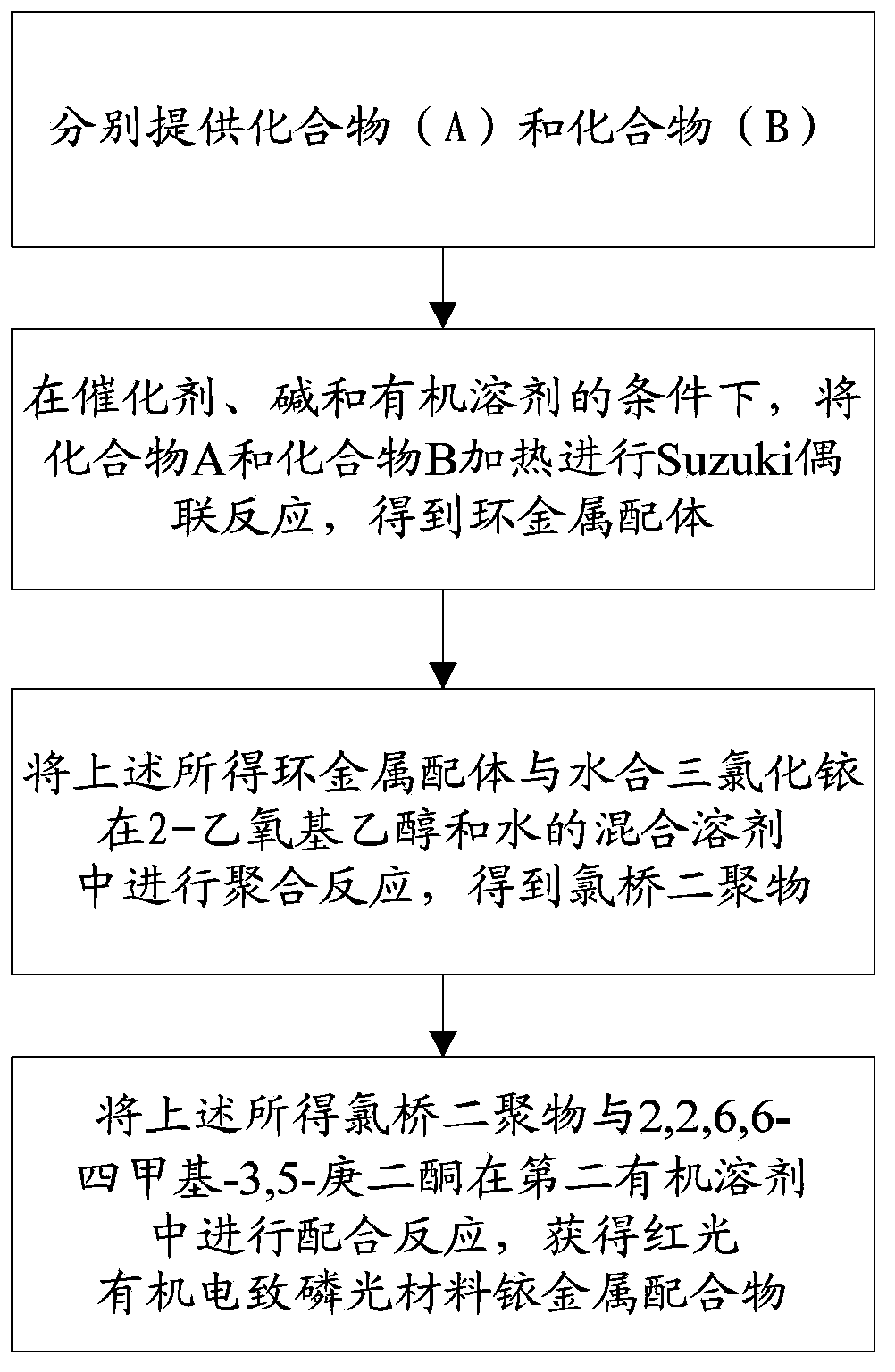
[0066] The preparation method of the above-mentioned red light organic electrophosphorescent material iridium metal complex comprises the following steps:
[0067] (1) Provide compound A and compound B1 represented by the following structural formula respectively:
[0068]
[0069] (2) Synthesis of cyclometal ligand 3-phenylcinnoline
[0070]
[0071] Under nitrogen protection, 0.83g (4.0mmol) 3-bromocinnoline (compound A), 0.59g (4.8mmol) phenylboronic acid (compound B1), 0.28g (0.24mmol) tetrakis (triphenylphosphine) palladium was dissolved in In 30mL of THF, 10mL of potassium carbonate aqueous solution with a mass fraction of 10% was added dropwise to the reaction system, heated to reflux temperature and stirred for 18h. After the reaction ...
Embodiment 2
[0092] Example 2: A red-light organic electrophosphorescent material iridium metal complex bis[3-(6'-methoxyphenyl)cinnoline-N,C 2 '](2,2,6,6-tetramethyl-3,5-heptanedione) iridium, as shown in the following structural formula:
[0093]
[0094] The preparation method of the above-mentioned red light organic electrophosphorescent material iridium metal complex comprises the following steps:
[0095] (1) Provide compound A and compound B2 represented by the following structural formula respectively:
[0096]
[0097] (2) Synthesis of cyclometal ligand 3-(2'-methoxyphenyl)cinnoline
[0098]
[0099] Under nitrogen protection, 0.83g (4.0mmol) 3-bromocinnoline, 0.61g (4.0mmol) 2-methoxyphenylboronic acid, 0.23g (0.20mmol) tetrakis (triphenylphosphine) palladium were dissolved in 25mL of toluene , 10 mL of 10% potassium carbonate aqueous solution was added dropwise to the reaction system, heated to reflux temperature and stirred for 10 h. After the reaction was completed...
Embodiment 3
[0119] Example 3: A red-light organic electrophosphorescent material iridium metal complex bis[3-(5'-ethoxyphenyl)cinnoline-N,C 2 '](2,2,6,6-tetramethyl-3,5-heptanedione) iridium, as shown in the following structural formula:
[0120]
[0121] The preparation method of the above-mentioned red light organic electrophosphorescent material iridium metal complex comprises the following steps:
[0122] (1) Provide compound A and compound B3 represented by the following structural formula respectively:
[0123]
[0124] (2) Synthesis of cyclometal ligand 3-(3'-ethoxyphenyl)cinnoline
[0125]
[0126] Under nitrogen protection, 0.83g (4.0mmol) 3-bromocinnoline, 0.80g (4.8mmol) 2-ethoxyphenylboronic acid, 0.28g (0.24mmol) dichlorobis (triphenylphosphine) palladium were dissolved in 40mL In THF, 20 mL of an aqueous solution of sodium carbonate with a mass fraction of 5% was added dropwise to the reaction system, and the reaction system was heated to reflux temperature and st...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com