Multi-metallic catalyst for catalyzing reforming reaction and preparation method of multi-metallic catalyst
A multi-metal catalyst and catalytic reforming technology, which is applied in the direction of naphtha catalytic reforming, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc., can solve the problems of deactivation and low activity , to achieve the effect of enhanced reforming performance and excellent catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
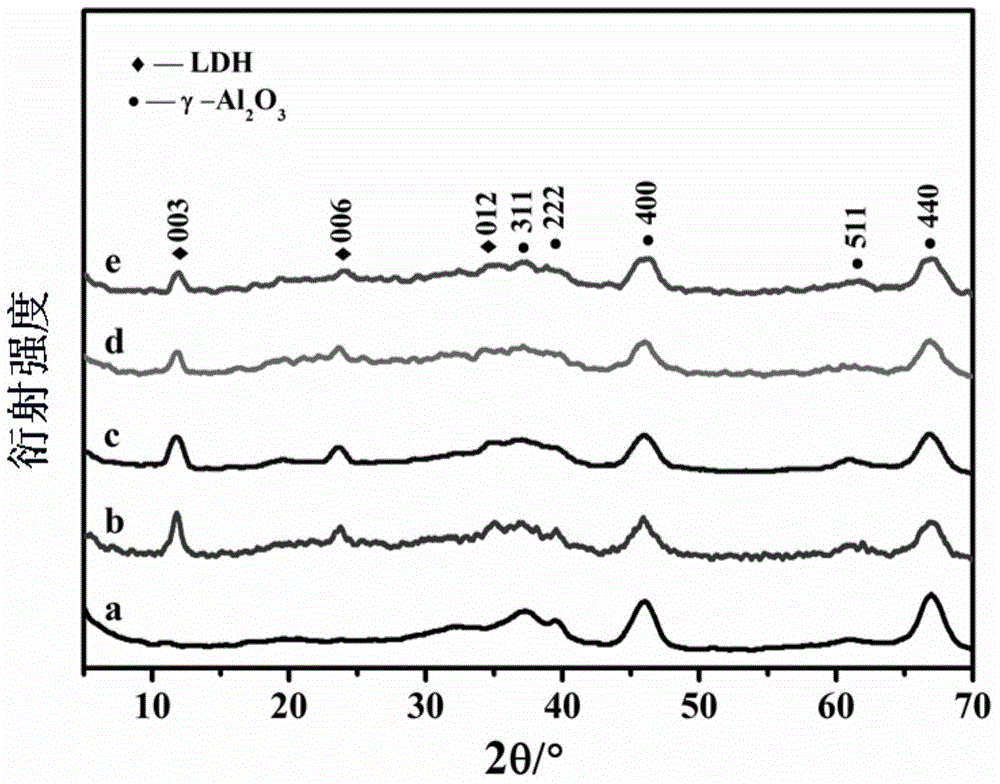
[0021] Step A: Configure Zn 2+ +Ni 2+ +Cu 2+ The concentration is 1.5mol / L, the molar ratio of Cu / Ni synthesized is 1:1, Ni 2+ The molar concentration of 0.3mol / L, the mixed solution of urea concentration 3mol / L, under the condition of vacuum, add 2g of dry γ-Al to the above mixed solution 2 o 3 , impregnated for 1 hour, transferred the above materials to an autoclave, crystallized at 100°C for 12 hours, filtered and washed with suction, 4 times with deionized water and 3 times with absolute ethanol, dried at 50°C for 12 hours, and the yield was about 2.30g.
[0022] Step B: 0.5g Ni-Cu-Zn-Al-CO 3 -LDHs / Al 2 o 3 Put it into the micro-reflector evaluation device, raise the temperature to 500°C at 5°C / min, and oxidize and heat for 4 hours in a dry air atmosphere with a flow rate of 60ml / min; 2 Flow rate 100ml / min purge 1h; H 2 Flow rate 60ml / min reduction 4h.
[0023] Step C: Utilize the micro-injection pump to pump n-heptane into it, and simultaneously adjust the pressu...
Embodiment 2
[0025] Step A: Configure Zn 2+ +Ni 2+ +Cu 2+ The concentration is 1.5mol / L, the molar ratio of Cu / Ni synthesized is 0.5:1, Ni 2+ The molar concentration of 0.3mol / L, the mixed solution of urea concentration 3mol / L, under the condition of vacuum, add 2g of dry γ-Al to the above mixed solution 2 o 3 , impregnated for 1 hour, transferred the above materials to an autoclave, crystallized at 100°C for 12 hours, filtered and washed with suction, 4 times with deionized water and 3 times with absolute ethanol, dried at 50°C for 12 hours, and the yield was about 2.30g.
[0026] Step B: Ni-Cu 0.5 -Zn-Al-CO 3 -LDHs / Al 2 o 3 (Cu / Ni ratio 0.5 / 1) supported Pt salt on the precursor, with Pt(C 5 h 7 o 2 ) 2 is the Pt precursor, acetone is the solvent, and the synthesized hydrotalcite precursor is dried. Under vacuum conditions, the prepared Pt solution of appropriate concentration is added to the dry carrier, and the hydrotalcite carrier is weighed. 1g, the Pt precursor is 0.0043 ...
Embodiment 3
[0030] Step A: Ni-Cu-Zn-Al-CO 3 -LDHs / Al 2 o 3 (Cu / Ni ratio 1 / 1) supported Pt salt on the precursor, with Pt(C 5 h 7 o 2 ) 2 is the Pt precursor, acetone is the solvent, and the synthesized hydrotalcite precursor is dried. Under vacuum conditions, the prepared Pt solution of appropriate concentration is added to the dry carrier, and the hydrotalcite carrier is weighed. 1g, the Pt precursor is 0.0043 g was dissolved in 0.2ml of acetone solvent, loaded by incipient wetness method, impregnated for 12h, and dried in an oven at 120°C to constant weight.
[0031] Step B: 0.5g Pt(C 5 h 7 o 2 ) 2 / Ni-Cu-Zn-Al-CO 3 -LDHs / Al 2 o 3 Put it into the micro-reflector evaluation device, raise the temperature to 500°C at 5°C / min, and oxidize and heat for 4 hours under the atmosphere of dry air at a flow rate of 60ml / min; 2 Flow rate 100ml / min purge 1h; H 2 Flow rate 60ml / min reduction 4h.
[0032] Step C: Use a micro-sampling pump to pump n-heptane, while adjusting the pressure ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com