A method for resolution and preparation of optically pure r-2-naphthylethylamine
A technology of R-2- and naphthylethylamine, which is applied in the field of resolution and preparation of optically pure R-2-naphthylethylamine, can solve the problems of high optical purity, difficult products, and high cost of asymmetric synthesis methods, and achieves high yields. The effect of high efficiency and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
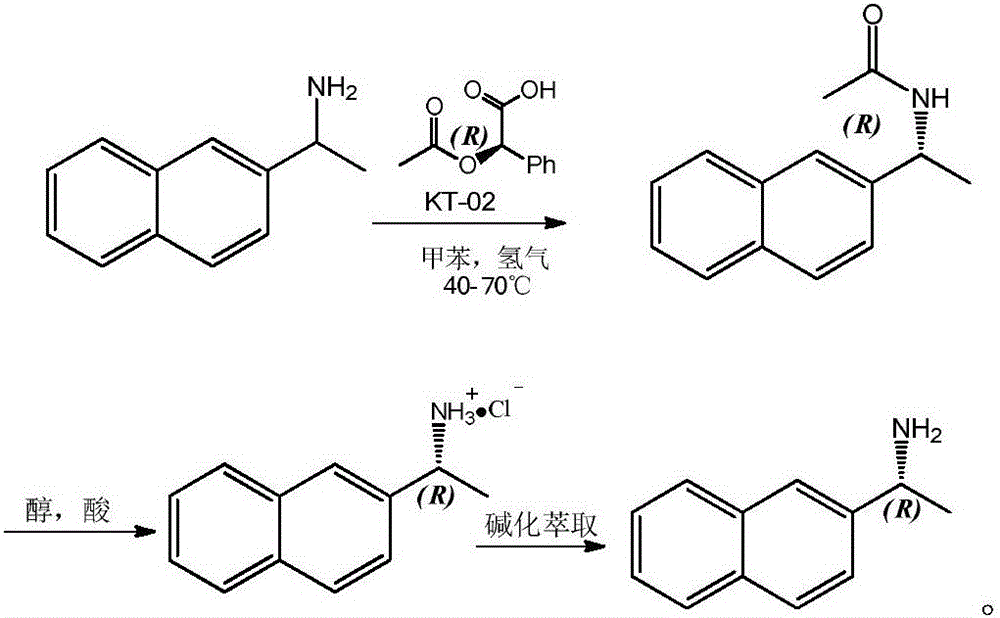
[0008] 1) Resolution and preparation of (R)-(1-(2-naphthyl)ethyl)acetamide
[0009] 500mL of toluene was added to a 1000mL autoclave as a solvent, followed by 85.5g of 2-naphthylethylamine, 90.2g of D-(-)-O-acetylmandelic acid, 5g of lipase novozym 435 and 8g of KT-02 (nickel catalyst ), after the addition is complete, seal the autoclave and replace the air in the autoclave with nitrogen, then inject hydrogen into the autoclave to a pressure of 1.0MP, start stirring, and raise the temperature to 60°C for reaction; after 20 hours, take a sample for detection, The disappearance of 2-naphthylethylamine is completely converted into (R)-(1-(2-naphthyl) ethyl) acetamide, and the product ee value reaches 99%; after the reaction finishes, the solution is concentrated, and then the volume ratio is A 10:1 mixed solvent of n-hexane and ethanol was subjected to column chromatography to obtain 102.3 g of pure (R)-(1-(2-naphthyl)ethyl)acetamide with a yield of 96%.
[0010] 2) Acid hydroly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com