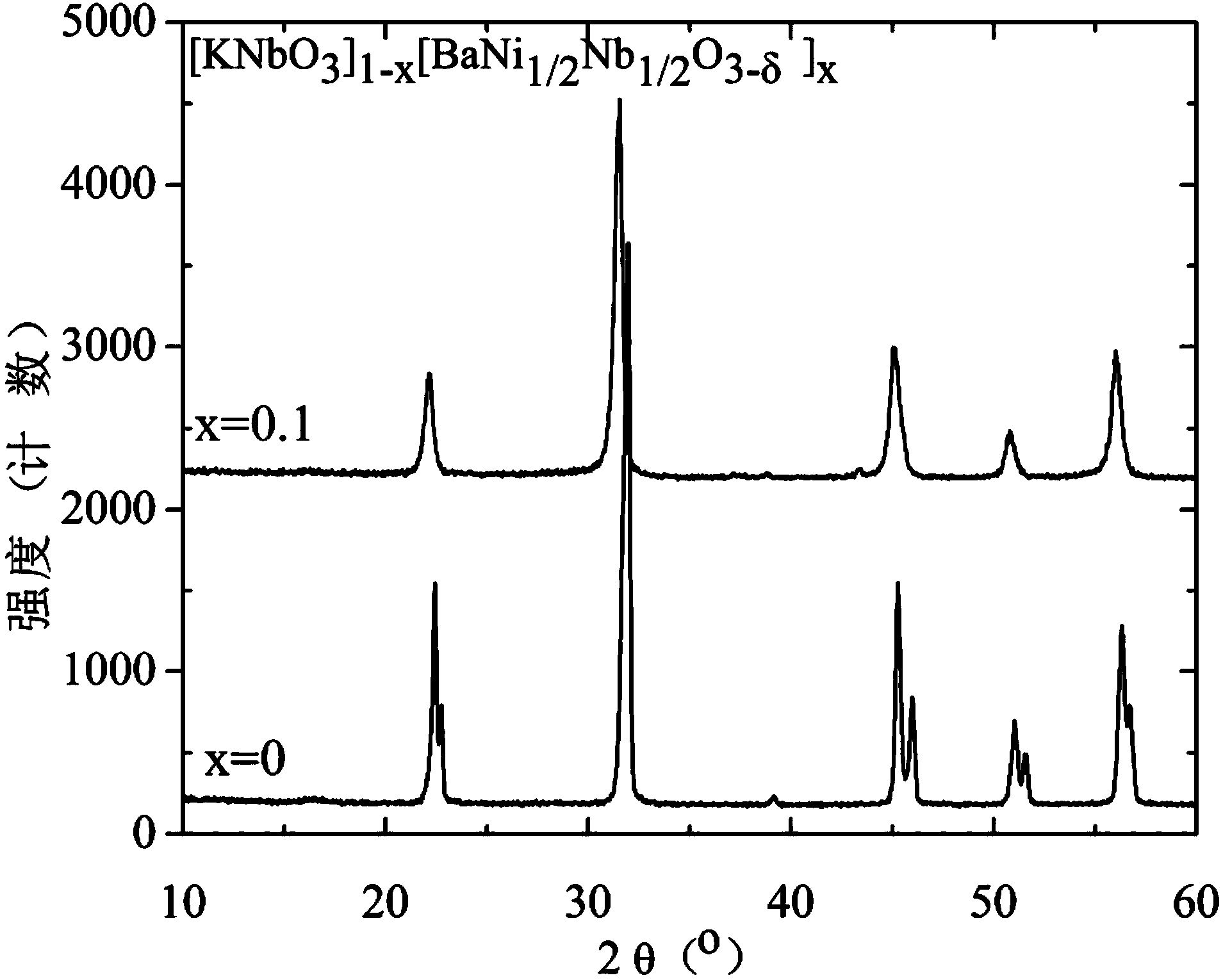
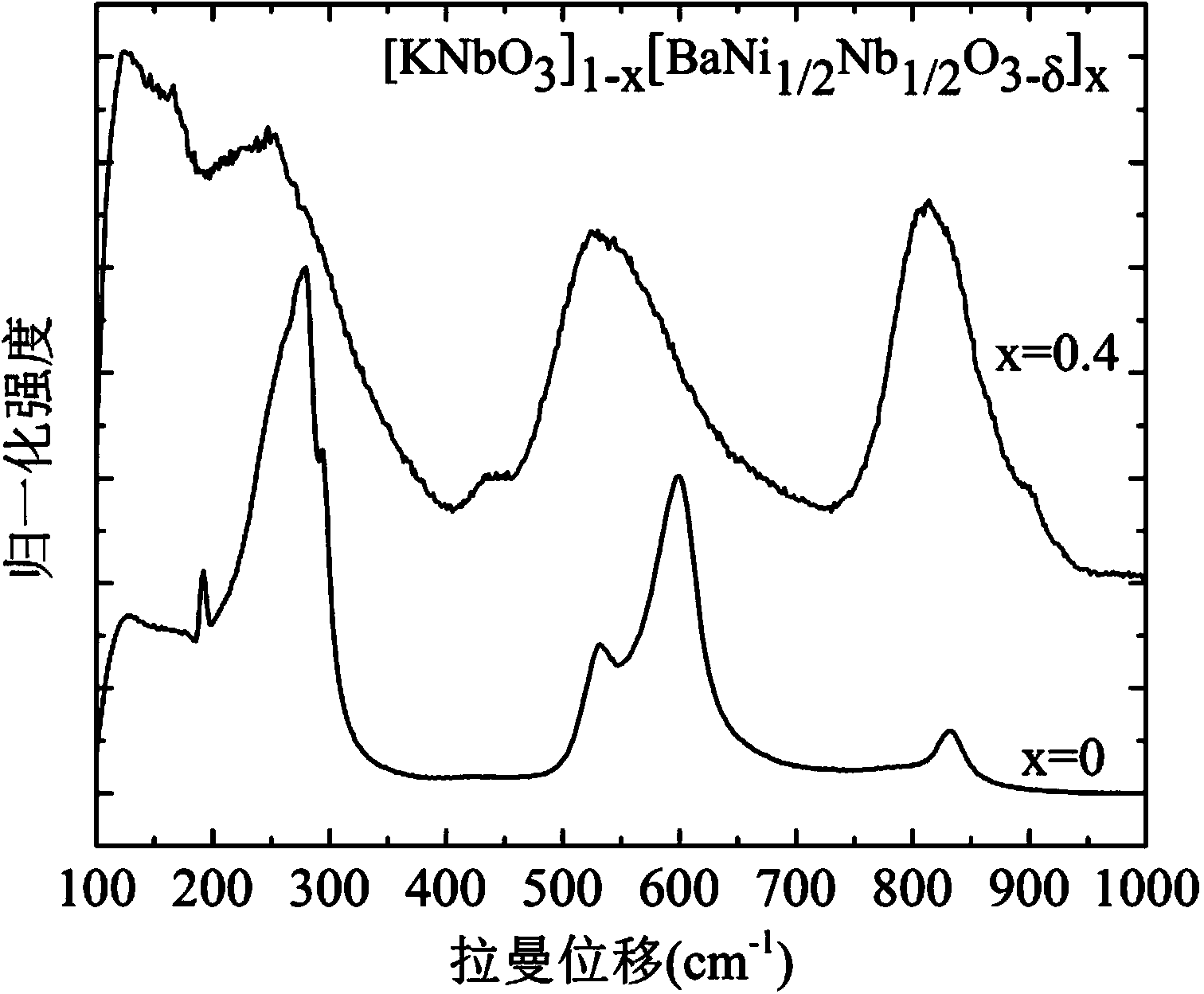
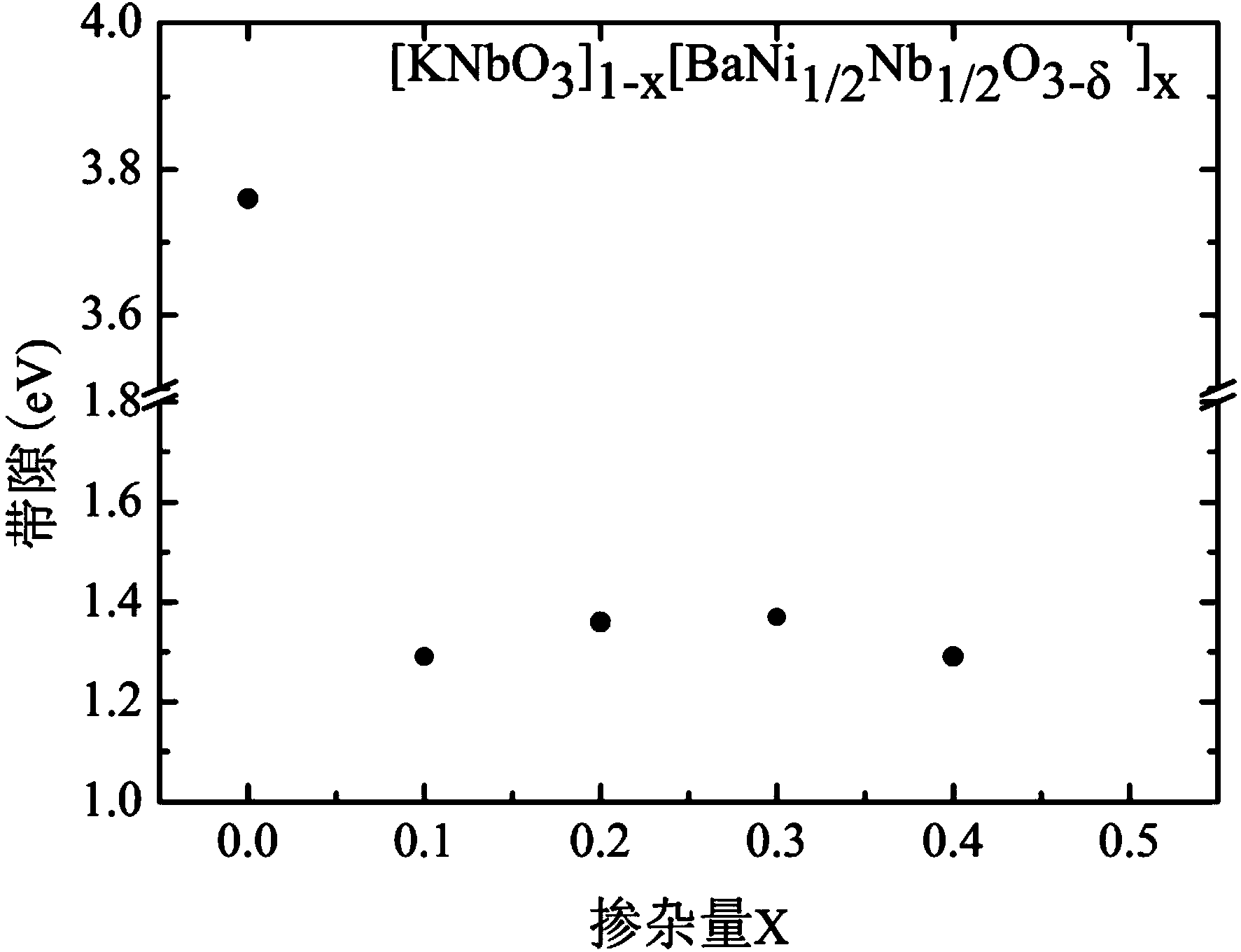
Preparation method of KNbO3 nano solid solution with adjustable optical band gap
An optical band gap and solid solution technology, applied in the field of preparation of KNbO3 nano solid solution, can solve the problems of poor controllability and high reaction temperature, and achieve the effect of low sintering temperature, low sintering temperature and complete crystallization of samples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 (corresponding to x=0.1):
[0035] Weigh 4.143g of citric acid monohydrate and 0.031g of basic nickel carbonate into 30ml of deionized water, and dissolve completely to form solution A; weigh 2.581g of niobium oxalate and add 30ml of deionized water to form a solution after completely dissolving B: Add solution B slowly to solution A drop by drop and stir thoroughly, then weigh 0.415g of anhydrous potassium carbonate and 0.099g of barium carbonate and add directly to the mixed solution of solution A and solution B, fully react and stir for 1h to form a colorless solution For transparent solution C, add 2.435 g of ethylene glycol at the end, stir well and then add 40 ml of deionized water to form 100 ml of mixed solution D. Put the mixed solution D in a constant temperature oil bath at 110°C to evaporate and promote the esterification of citric acid and ethylene glycol, and finally obtain a viscous gel, which is placed in a vacuum drying oven at 180°C for pr...
Embodiment 2
[0036] Embodiment 2 (corresponding to x=0.4):
[0037] Weigh 7.07g of citric acid monohydrate and 0.126g of basic nickel carbonate into 50ml of deionized water, and dissolve completely to form solution A; weigh 2.173g of niobium oxalate and add 30ml of deionized water to form a solution after completely dissolving B: Add solution B slowly to solution A drop by drop and stir thoroughly, then weigh 0.311g of anhydrous potassium carbonate and 0.395g of barium carbonate and add directly to the mixed solution of solution A and solution B, fully react and stir for 1h to form a colorless solution Transparent solution C; finally add 4.154g of ethylene glycol, stir well and add 20ml of deionized water to form 100ml of mixed solution D. Put the mixed solution D in a constant temperature oil bath at 120°C to heat and evaporate and promote the esterification of citric acid and ethylene glycol, and finally obtain a viscous gel, which is placed in a vacuum drying oven at 200°C for processin...
Embodiment 3
[0039] Embodiment 3 (corresponding to x=0.5):
[0040] Weigh 8.042g of citric acid monohydrate and 0.157g of basic nickel carbonate into 60ml of deionized water, and dissolve completely to form solution A; weigh 2.038g of niobium oxalate and add 30ml of deionized water to form a solution after completely dissolving B; Add solution B slowly to solution A drop by drop and stir thoroughly, then weigh 0.276g of anhydrous potassium carbonate and 0.493g of barium carbonate and add directly to the mixed solution of solution A and solution B, fully react and stir for 2 hours to form a colorless Transparent solution C; finally add 4.727g of ethylene glycol, stir well and add 10ml of deionized water to form 100ml of mixed solution D. Put the mixed solution D in a constant temperature oil bath at 130°C to heat and evaporate and promote the esterification of citric acid and ethylene glycol, and finally obtain a viscous gel, which is placed in a vacuum drying oven at 220°C for 3 hours A d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com