Process for preparing rebaudioside M
A process method and reaction technology, applied in the field of drug synthesis, can solve the problems of difficult realization, complicated operation, high cost and the like, and achieve the effects of good environmental protection effect, simple operation and low cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
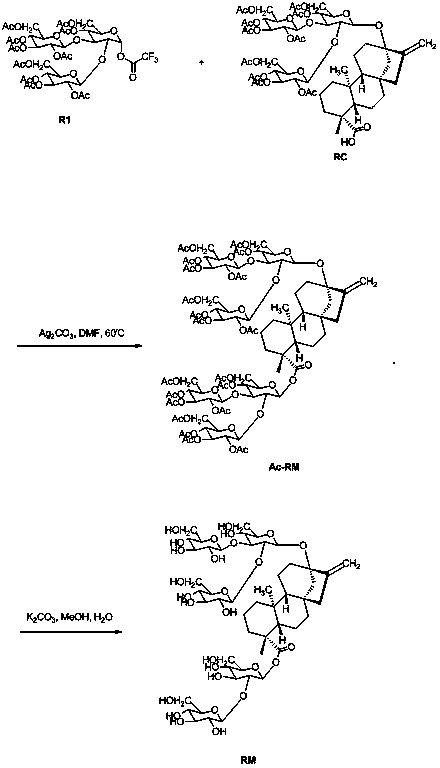
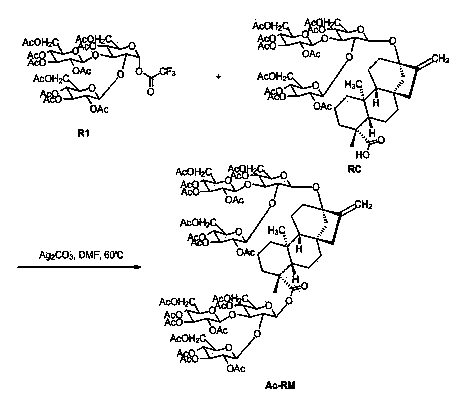
[0015] Provide a kind of preparation method of Ac-RM, reaction process and reaction equation are as follows:
[0016]
[0017] Compound R1 (1.2 g, 1.2 mmol), Ag 2 CO 3 (0.55 g, 2 mmol) was added to DMF (20 mL), heated to 30-100 °C and compound RC (1.2 g, 1 mmol) was added slowly, and the reaction system was stirred overnight at 30-100 °C. Cool to room temperature, filter, add ethyl acetate (80 mL) to the filtrate, wash with saturated brine (30 mL*3), dry over anhydrous sodium sulfate, spin dry, and use a silica gel column (ethyl acetate:petroleum ether=1 :10 to 1:2) to obtain compound Ac-RM (0.7 g, 32%). m / z: 2148 (M + NH 4 ) + .
Embodiment 2
[0019] Provide a kind of preparation method of Ac-RM, reaction process and reaction equation are as follows:
[0020] Compound Ac-RM (0.7 g, 0.32 mmol) was dissolved in methanol (10 mL) at room temperature, and K 2 CO 3 (0.14 g, 1 mmol), and the reaction system was stirred overnight at room temperature. After cooling to room temperature, the reaction solution was neutralized to pH = 6 with 1N hydrochloric acid solution, concentrated, and recrystallized with ethanol / water to obtain 0.21 g of white solid rebaudioside M (RM), with a yield of 58%. m / z: 1146 (M + NH 4 ) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com