Subcutaneous injection administration system based on Brij97 liquid crystal and preparation method of subcutaneous injection administration system based on Brij97 liquid crystal
A technology for subcutaneous injection and drug delivery system, which is applied to pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. To achieve the effect of convenient and easy process control, increase patient compliance, and increase stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Weigh 200 mg Brij97, 50 mg IPM, 50 mg water, and 1.5 mg insulin (the amount of IPM accounts for about 20% of the total amount of Brij97 and IPM). Put the weighed Brij97 and IPM into the same vial, heat and melt in a water bath at 40°C to become liquid, and vortex to mix. Prepare insulin solution. Add the insulin solution to Brij97 and IPM, vortex to mix, and stand at room temperature to remove air bubbles.
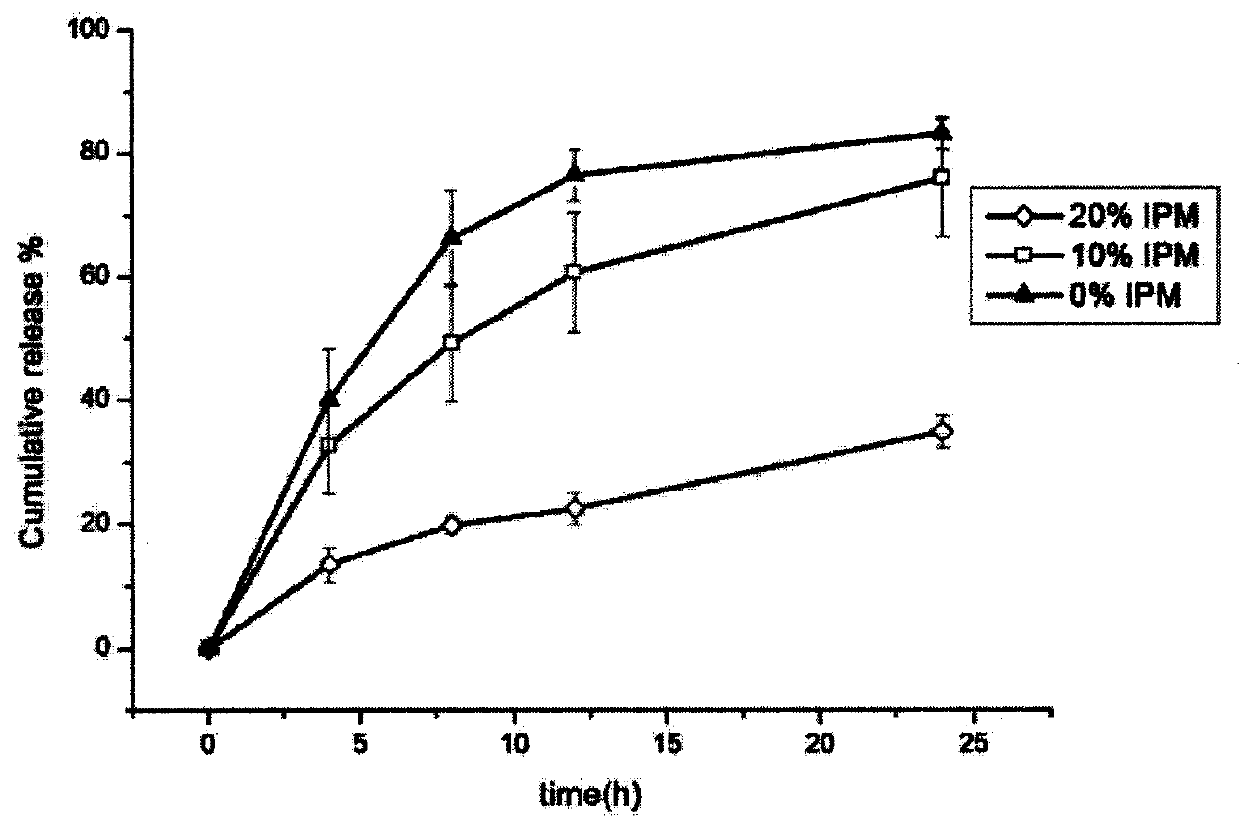
[0035] For the 24h in vitro release of the Brij97 liquid crystal subcutaneous injection drug delivery system prepared in this example, see figure 1 .
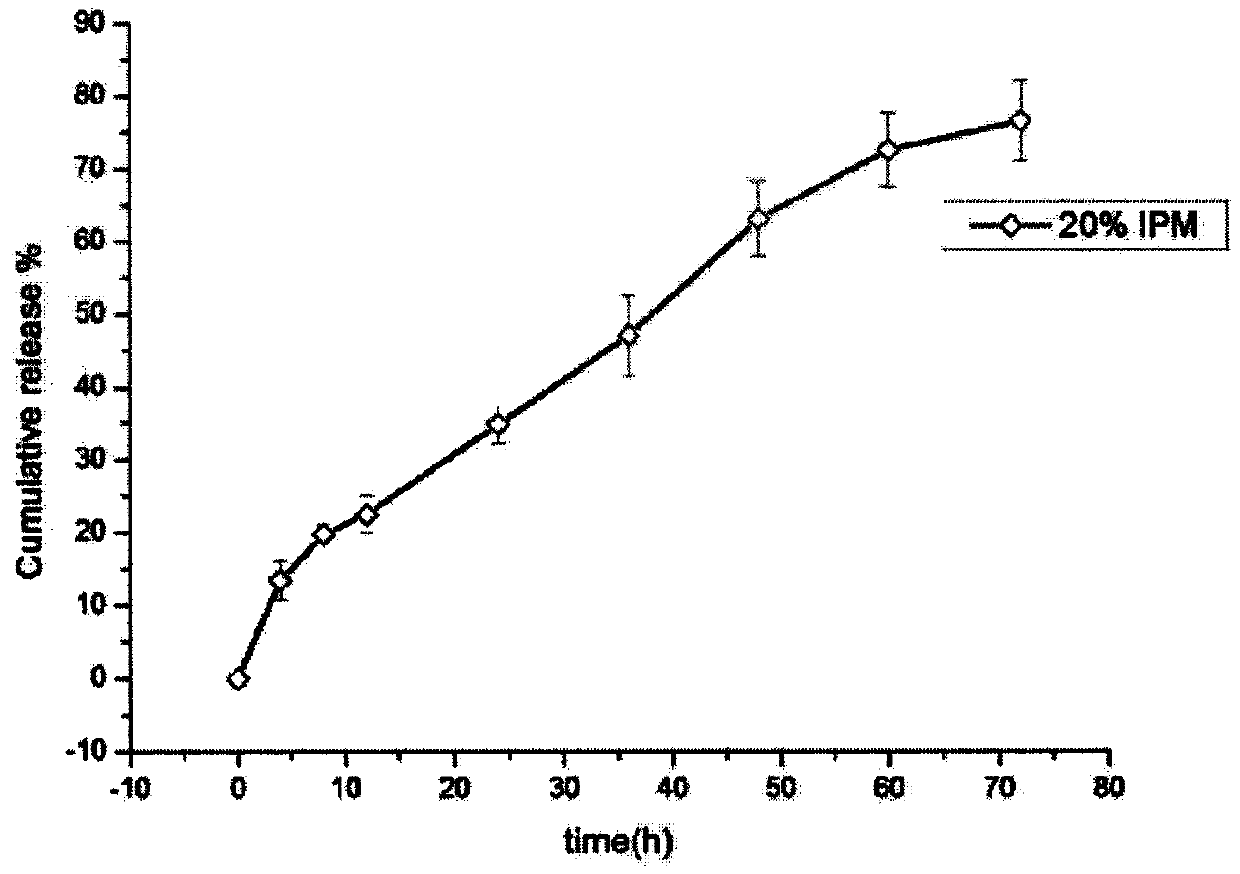
[0036] The 72h in vitro release of the Brij97 liquid crystal subcutaneous injection drug delivery system prepared in this example is shown in figure 2 .
[0037] Polarizing micrographs (25°C, magnification 200) of the subcutaneous injection drug delivery system of Brij97 liquid crystal prepared in this example after contacting water for 0.5h, see image 3 . image 3 Appears as a lamellar phase.
[0038] Polar...
Embodiment 2
[0042]Weigh 210mg Brij97, 90mg water, 1.5mg insulin. Put the weighed Brij97 and IPM into the same vial, heat and melt in a water bath at 40°C to become liquid, and vortex to mix. Prepare insulin solution. Add the insulin solution to Brij97 and IPM, vortex to mix, and stand at room temperature to remove air bubbles.
[0043] For the 24h in vitro release rate of the Brij97 liquid crystal subcutaneous injection drug delivery system prepared in this example, see figure 1 .
[0044] For the viscosity of the subcutaneous injection drug delivery system of the Brij97 liquid crystal prepared in this example, see Image 6 .
Embodiment 3
[0046] Weigh 235 mg Brij97, 25 mg IPM, 40 mg water, and 1.5 mg insulin (the amount of IPM accounts for about 10% of the total amount of Brij97 and IPM). Put the weighed Brij97 and IPM into the same vial, heat and melt in a water bath at 40°C to become liquid, and vortex to mix. Prepare insulin solution. Add the insulin solution to Brij97 and IPM, vortex to mix, and stand at room temperature to remove air bubbles.
[0047] For the 24h in vitro release rate of the Brij97 liquid crystal subcutaneous injection drug delivery system prepared in this example, see figure 1 .
[0048] For the viscosity of the subcutaneous injection drug delivery system of the Brij97 liquid crystal prepared in this example, see Image 6 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com