Long-acting recombinant human chorionic gonadotropin fusion protein
A technology of fusion protein and amino acid, applied in the fields of molecular biology and medicine, can solve the problems of short half-life in vivo, difficult purification, low expression of recombinant HCG, etc., and achieve the effects of improving biological activity, prolonging stable protein, and reducing toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1. Construction of gene expression vectors encoding recombinant HCG-Fc fusion proteins
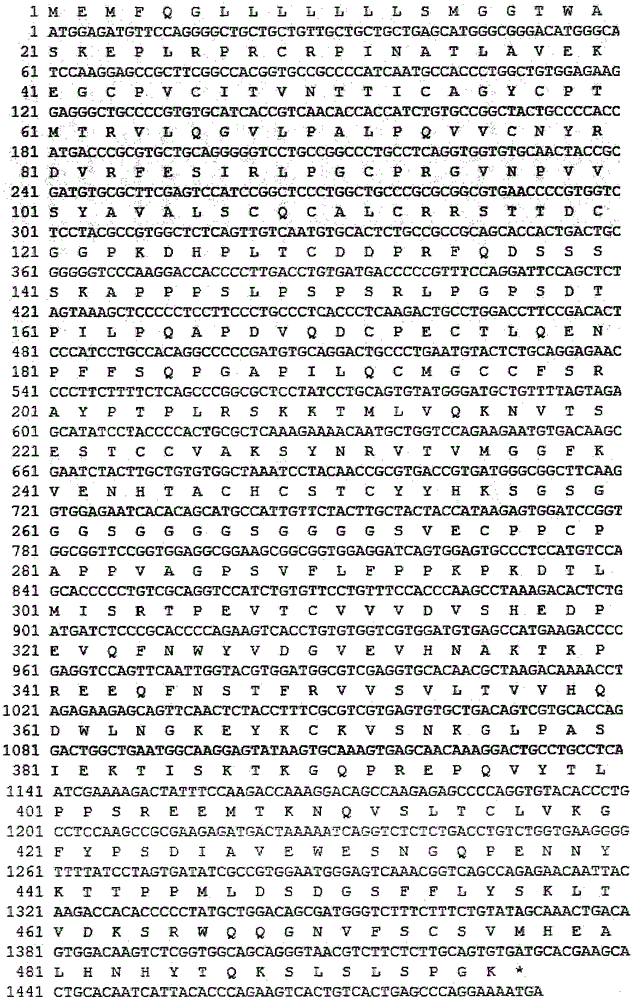
[0063] The gene sequence design was optimized based on the preferred codons of CHO cells, and the optimized fusion gene containing the signal peptide encoding the β chain of HCG protein and its mature peptide and the mature peptide of the HCG α chain was synthesized by artificial synthesis. The 756bp DNA fragment was inserted between the EcoRV restriction sites in the transfer vector such as pUC57 to obtain the HCG plasmid (pHCG), and the correctness of the inserted sequence was verified by DNA sequencing.
[0064] Fusion gene L encoding flexible peptide linker (Linker, detection "L") and Fc variants (vIgG2Fc, vIgG4Fc and vIgG1Fc) fragments containing BamHI (5' end) and EcoRI (3' end) restriction sites were artificially synthesized - vIgG2Fc, L-vIgG4Fc and L-vIgG1Fc. The obtained fusion gene fragments were respectively inserted between the BamHI and EcoRI sites of a transfe...
Embodiment 2
[0067] Example 2. Stable expression of recombinant HCG-Fc fusion protein in mammalian cells
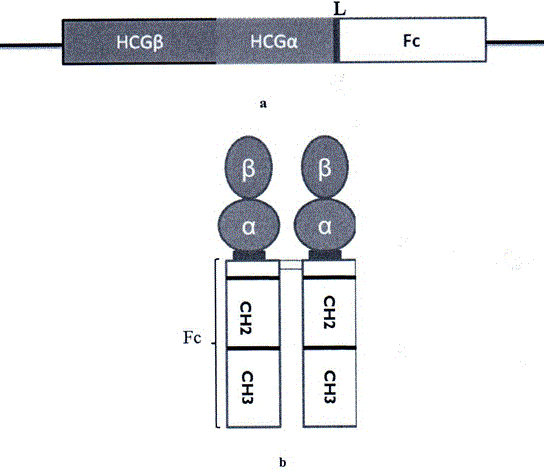
[0068] The expression plasmid pCDNA3-HCG-L-Fc constructed in Example 1 was transfected into DHFR enzyme-deficient CHO host cells (CHO-DHFR - ), figure 2 b shows a schematic diagram of the recombinant dimerized HCG-Fc fusion protein. Transfection was carried out by electroporation, using a GenePulser Electroporator (Bio-Rad Laboratories, Hercules, CA) with a capacitance of 960 μFd, setting its electric field to 250 V, and using 2 to 5 × 10 cells in the cuvette. 7 Add 10 μg of plasmid DNA linearized with PvuI to each cell. Two days after transfection, the medium was changed to a growth medium containing 100 μg / mL Zeocin resistance marker gene to obtain transfectants that had passed the primary resistance screening. Using westemblotting method, use anti-HCG antibody to detect the expression of HCG-Fc, such as Figure 8 . The use of DHFR to amplify the selectable marker gene increas...
Embodiment 3
[0069] Example 3. Production and purification of recombinant HCG-Fc fusion protein
[0070] Using the high-yield cell line obtained in Example 2, firstly carry out serum-free acclimatization culture in a petri dish, then transfer to a shake flask for suspension acclimatization culture, during the acclimatization process, the medium is screened at the same time, and different components are added to observe the growth of the cells. Growth state, growth trend, and biochemical indicators such as the activity of the expressed product and sialic acid, the preferred cell culture conditions are: adding 100 μM Cu to the basal medium 2+, adding 2mM ManNAc (N-acetyl-D-aminomannose) to the feeding medium, this method can increase the glycosylation degree of the recombinant HCG-Fc fusion protein, and increase the sialic acid content by about 20%. After the acclimatization is successful, the cells are expanded to a sufficient amount, and the 7L bioreactor is monitored for culture. When the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com